Full Text
Introduction
Benign bronchoesophageal fistulas (BEF) are uncommon and usually identified in neonates. Adult bronchoesophageal fistulas are typically acquired and could be of malignant, traumatic, or infectious origin. The non-malignant causes of acquired BEF are blunt or penetrating trauma, granulomatous infections as tuberculosis, previous surgery of trachea and oesophagus, corrosive fluid ingestion, poisons and inhalation burns, HIV infection, iatrogenic etc.
Road-traffic accidents, resulting in chest wall crush injuries as a result of steering wheel impact, cause the majority of traumatic fistulae [1-3]. Compression of the trachea and oesophagus between the sternum and the thoracic spine results in laceration and disruption of blood supply. This causes a delay in presentation of 3–10 days whilst necrosis develops [1]. Although much less common, a more acute presentation can occur as a result of traumatic tracheal and oesophageal rupture. The TOF, which form mostly in the carinal area, has a reported operative mortality of 15%, predominately because of the rapid spread of mediastinal infection [1]. Acquired BEF can occur as a consequence of tuberculosis, HIV infection and mediastinitis. Infection as an aetiological factor in acquired BEF has declined in recent years [1-3].
In order to isolate the BEF and prevent pulmonary aspiration, oesophageal stenting can be undertaken. The stent is usually placed endoscopically under conscious sedation, thus negating the need for a general anaesthetic. Early stents were rigid plastic tubes that required aggressive oesophageal dilatation with the risk of tearing the BEF. The stents themselves were prone to migration, obstruction and were painful. Modern self-expanding metallic stents require no dilatation and are better tolerated but they can also lead to late complications as recurrent dysphagia due to reobstruction from tumor or food impaction, migration, tracheoesophageal fistula, bleeding, gastroesophageal reflux disease/ aspiration [4, 5].
Case reports
Case 1
A 27 year-old male met with road traffic accident 3 years back, sustained multiple fractures i.e. fracture shaft of right femur, intercondylar fracture of left femur, fracture of left radius and fracture patella. He was intubated and was detected to have gurgling sound from naso gastric tube. Fibrooptic bronchoscopy revealed a fistula between oesophagus and the trachea 1 cm. above the carina. CECT and virtual bronchoscopy confirmed the fistula. An esophageal plastic stent was placed endoscopically to start with which, later was exchanged to a self-expanding plastic stent. The stent however, was successful only for a few weeks. Later it had to be repeatedly repositioned as it was not remaining in place and he was not able to eat because of constant aspiration. He developed chronic aspiration pneumonitis and as he was getting rapidly malnourished, a feeding jejunostomy was done. After his nutrition was partly restored, definitive surgery was planned. Direct repair of the fistula which by now had extended to 1.5 cm and in to the left bronchus was considered too risky. Therefore it was decided to defunctionalize the oesophagus by stapling it in the neck and at the GE junction after intra-operative removal of the stent. However, the stent remained firmly stuck and could not be removed by operative enteroscopy (Figure 1). It had to be left in situ and a retrosternal gastric pull-up was done after stapling the oesophagus in the neck and at the GE junction, one year after the fistula had formed.
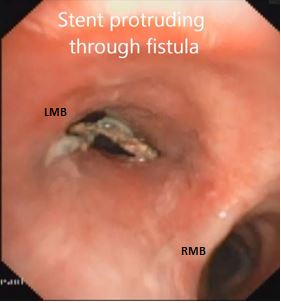
Figure 1: Bronchoscopy showing oesophageal stent through the fistula.
Although post-operative recovery was good and he had a good feeding access, he continued to have cough and pneumonitis from the constantly leaking oesophageal secretions. Bronchoscopy showed non healing fistula in the carinal region and pus pouring into left lung from the mucocele of the native oesophagus. Physiotherapy and appropriate antibiotics did not help and he had to be reoperated. This time a thoraco-abdominal incision was used. The oesophagus was opened bit by bit and the stent was removed piecemeal without disturbing the fistula. The collapsed oesophagus was stapled close to the fistula on either side leaving a three cm remnant to patch the fistula. He recovered well and at two year follow-up was symptom free and had gained twenty kilos of weight.
Case 2
A 59-year female patient presented to KIMS with severe cough on deglutition, inability to swallow even saliva and with a feeding jejunostomy in place.
She was diagnosed to have pulmonary Koch's with mediastinal lymphadenitis 3 years back with positive sputum for AFB and had a full course of ATT. But the symptoms were not completely relieved and she developed dysphagia and irritant cough. Initial endoscopy showed deep esophageal ulcer with diverticulum. Barium swallow showed a fistulous communication between esophagus and left bronchus (Figure 2), which was confirmed on CT scan and also showed multiple caseous lymphnodes around it. Second upper GI endoscopy showed a large broncho-esophageal fistula track at 27 cms from incisors. Feeding jejunostomy was done and she was observed for 3 months. A self-expandable covered metallic stent (NITI-S) was then deployed in the esophagus at the site of fistula.
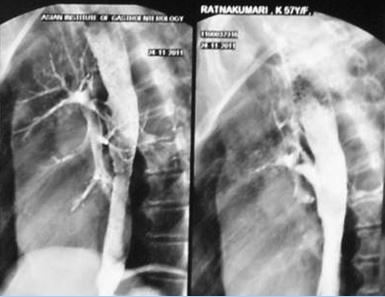
Figure 2: Barium Swallow showing fistula between oesophagus and left bronchus.
She tolerated oral soft diet and was asymptomatic for 2 months. Then she developed similar symptoms and the stent was found to have slipped (Figure 3). Repositioning was only successful for a few days and the procedure had to be repeated three times over three months. A second self expandable metallic stent was then placed through the previous stent. After the second stent placement, she again had cough with oral feeds and a weight loss of 40 kilos. She did not agree for a third stent placement and consulted us at KIMS.
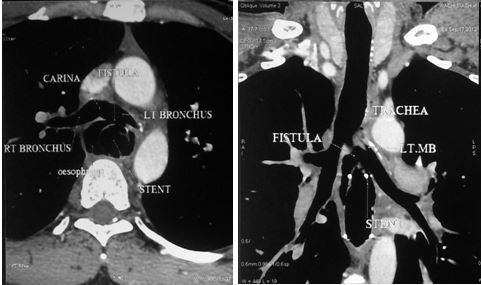
Figure 3: Reconstructed images of CT showing fistula due to stent erosion.
A complete evaluation confirmed a large left broncho-esophageal fistula with two self-expanding stents in place (Figure 4). Operative removal of the stents, oesophageal stapling on either side of the fistula, oesophagectomy of the residual oesophagus and retrosternal gastric by-pass were done (Figure 5). Post operatively the patient recovered well and was asymptomatic. Gastrograffin study showed free flow of contrast. Patient was discharged on 7th POD on oral feeds and is doing well at one-year follow-up.
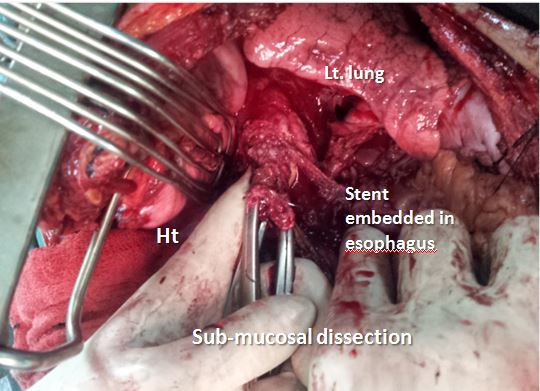
Figure 4: Operative picture showing the firmly embedded stent and submucosal dissection to extract the stent.
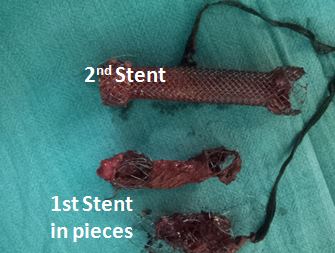
Figure 5: The extracted stents in pieces.
Case 3
A 55 year-old female presented to the hospital with a left broncho-oesophageal fistula and chronic aspiration pneumonitis. She gave history of accidental swallowing of a denture and endoscopic extraction following repeated attempts.
She was evaluated with CECT, Bronchoscopy and the fistula was confirmed. After a vigorous preoperative chest physiotherapy and antibiotic management she was operated. Esophageal exclusion, gastric pull-up and feeding jejunostomy were done as in previous two cases with a successful result. She is on regular follow up and has remained asymptomatic.
Discussion
In adults, a fistula between the oesophagus and the respiratory tract is usually the result of malignant disease. Non-malignant oesophageal respiratory fistulas are relatively rare and may be congenital or acquired from trauma [6]. A BEF is typically associated with repeated and persistent respiratory infections, which can lead to the development of bronchiectasis and coughing bouts when eating [7]. Hemoptysis is also seen occasionally. The presence of food in the sputum or choking on swallowing liquids may lead to the diagnosis of BEF. More uncommon symptoms include dysphasia, epigastric discomfort and reflux caused by the stomach filling with air on expiration.
Division of the fistula and excision of any permanently damaged lung segment have been considered the method of choice in the treatment of this disease. Other treatment modalities cited in the literature were local application of sodium hydroxide, acetic acid and using a surgical stapler [8, 9]. Immediate surgical treatment is recommended for a finding of BEF despite its seemingly benign nature. Surgical treatment has the most favorable prognosis, but endoscopic occlusion with silver nitrate, biological glue, or a Celestin tube is also an option if a patient cannot tolerate a surgical procedure [10].
Song HY et al. reported stent migration in 7 of 12 patients (58 %), and new stricture formation was seen in 50 % of the patients [11]. Data regarding the use of SEMSs in benign conditions are in the form of case series and case reports. SEMSs placed for benign disease are associated with significant complications, such as high migration rates, bleeding, fistula, erosion into vital structures, recurrent strictures, and death [14, 15]. As a matter of fact only self-expandable plastic stents are currently approved by the FDA for the treatment of benign disease and expandable metal stents are not approved [12, 13].
In a retrospective analysis using partially covered SEMSs for benign indications that included eight patients with esophageal stent placement, half of the patients had major complications. Review of 29 patients in whom partially covered SEMSs were placed for benign esophageal strictures, new stricture formation was seen in 41 % , stent migration in 31 % , chest pain or reflux in 21 % , tracheo-esophageal fistula in 6 % , and anemia in 3 % of the patients . Thus, the overall major complication rates associated with SEMSs from the available uncontrolled data may be as high as 80 % [16].
In the three patients described above, attempts to remove the displaced and impacted stents were not successful. In the first case when the operative attempt to remove the stent through the neck and abdominal incisions did not succeed, the esophagus was stapled in the neck and at the GE junction and a retrosternal gastric conduit was created. However the patient continued to aspirate the esophageal secretions in to the lungs through the fistula that was kept open by the retained stent. This problem was addressed by a thoraco-laparotomy, direct incision over the stent, removal of the self-expanding plastic stent piecemeal and stapling the collapsed esophagus on either side of the fistula, leaving the small collapsed esophageal remnant to bridge the communication (Figure 6). Following success of this manoeuvre, the same technique was applied primarily to the next two patients with successful results. At a follow-up of 1-3 years, all the three patients are symptom free.
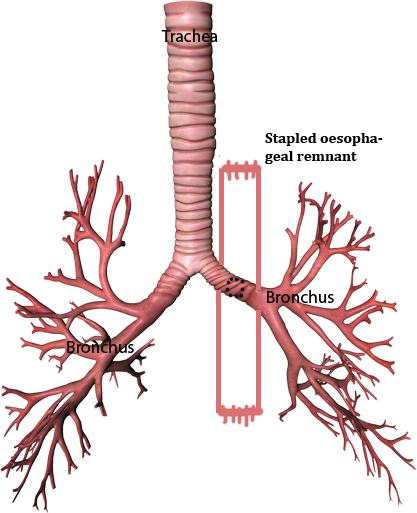
Figure 6: Stapling the collapsed esophagus on either side of the fistula.
Conclusion
The three cases described highlight the morbidity of self-expanding stents in the treatment of benign oesophageal problems. Apart from stent migration and difficulties in extracting the stents after their purpose is served, if left for unduly long periods, they may cause pressure necrosis and fistulization between surrounding aero-digestive tracts. As broncho-oesophageal fistulas are difficult to manage, they require multidisciplinary approach, correct judgement of the clinical problem in a given patient and innovative surgical approach.
Acknowledgements
Acknowledgements are due to Department of Radiology and Imaging, KIMS, Secunderabad, Telangana, India.
Conflict of interest
The authors declare no conflict of interest.
References
1. Reed MF, Mathisen DJ. Tracheoesophageal fistula. Chest Surg Clin N Am. 2003; 13:271–289.
2. Couraud L, Ballester ML, Delaisement C. Acquired tracheoesophageal fistula and its management. Semin Thorac Cardiovasc Surg. 1998; 8:392–399.
3. Grillo HC. Acquired tracheoesophageal fistula and bronchoesophageal fistula. In: Grillo HC, ed. Surgery of the Trachea and Bronchi. New York: BC Dekker Inc., 341–56.
4. Papachristou GI, Baron TH. Use of stents in benign and malignant esophageal disease. Rev Gastroenterol Disord 2007; 7:74–88.
5. Ackroyd R, Watson DI, Devitt PG, Jamieson GG. Expandable metallic stents should not be used in the treatment of benign esophageal strictures . J Gastroenterol Hepatol 2001; 16: 484–487.
6. Gerzic Z, Rakic S, Randjelovic T. Acquired benign esophagorespiratory fistula. Report of consecutive cases. Ann Thorac Surg. 1990; 50:724–727.
7. Osinowo O, Harley HR, Janigan D. Congenital broncho-oesophageal fistula in the adult. Thorax. 1983; 38:138–142.
8. Parry W, Juma A. Congenital bronchoesophageal fistula. Ann Thorac Surg. 1991; 51:346–347.
9. Weissberg D, Kaufman M. Bronchoesophageal fistula in adults: Congenital or acquired? J Thorac Cardiovasc Surg. 1990; 99(4):756–757.
10. Cossentino MJ, Ormseth EJ, Tavaf-Motamen H, Cheney CP. Congenital bronchoesophageal fistula in the adult: a case report. Am J Gastroenterol. 2000; 95(8):2116–2118.
11. Song HY, Park SI, Do YS, Yoon HK, Sung KB, et al. Expandable metallic stent placement in patients with benign esophageal strictures: results of long term follow-up. Radiology 1997; 203(1):131–136.
12. Sharma P, Kozarek R. Practice Parameters Committee of American College of Gastroenterology. Role of esophageal stents in benign and malignant diseases. Am J Gastroenterol 2010; 105(2):258–273.
13. Schubert D, Scheidbach H, Kuhn R, Wex C, Weiss G, et al. Endoscopic treatment of thoracic esophageal anastomotic leaks by using silicone-covered, self-expanding polyester stents. Gastrointest Endosc. 2005; 61(7):891–896.
14. Papachristou GI, Baron TH. Use of stents in benign and malignant esophageal disease. Rev Gastroenterol Disord 2007; 7(2):74–88.
15. Ackroyd R, Watson DI, Devitt PG, Jamieson GG. Expandable metallic stents should not be used in the treatment of benign esophageal strictures. J Gastroenterol Hepatol. 2001; 16:484–487.
16. Sandha GS, Marcon NE. Expandable metal stents for benign esophageal obstruction. Gastrointest Endosc Clin N Am. 1999, 9(3):437–446.