Original Research
2018
September
Volume : 6
Issue : 3
Clinical outcome of the axilla after axillary radiation in breast cancer: A retrospective study from MNJIO & RCC Hyderabad
Raman RR
Pdf Page Numbers :- 63-67
Raghu Raman R1,*
1MNJ Institute of Oncology & Regional Cancer Centre, Hyderabad, Telangana, India
*Corresponding author: Dr. R. Raghu Raman, Associate Professor, MNJ Institute of Oncology & Regional Cancer Centre, Red Hills, Lakadikapul, Hyderabad-500004, Telangana, India. Email: raghuraman3008@gmail.com
Received 28 March 2018; Revised 17 May 2018; Accepted 30 May 2018; Published 07 June 2018
Citation: Raman RR. Clinical outcome of the axilla after axillary radiation in breast cancer: A retrospective study from MNJIO & RCC Hyderabad. J Med Sci Res. 2018; 6(3):63-67. DOI: http://dx.doi.org/10.17727/JMSR.2018/6-11
Copyright: © 2018 Raman RR. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim: To study the axillary recurrences after calculation of radiation dose delivered to the mid axillary plane in breast cancer patients receiving adjuvant radiation.
Materials: 208 patients (148 cobalt, 60 linear accelerator) attending the follow up clinics from May 2007 to Dec 2012, had their axillary separation measured at the first visit and the dose to mid plane of axilla was extrapolated from PDD’s.
Results: The actual mean dose received by the axilla was in the cobalt arm 4058.9 cGy (range 3301cGy to 4705cGy) and 4134.77 cGY (range 3597cGy to 4824cGy) in the LA arm, which is less than the usual recommended dose of 4500cGy to 5000cGy to midplane. The doses were subclinical as posterior axillary boost was not planned. There were 10 recurrences including only 1 axillary recurrence (0.48%).
Discussion: The role of PMRT in breast cancer is well established and so is the morbidity of combined axillary dissection and radiation. The adoption of certain techniques of planning can result in sub optimal doses to the axillary mid plane. However suboptimal dosing may not affect the outcome of axillary recurrences.
Conclusion: The incidence of axillary recurrences in a heterogeneous population receiving locoregional post-mastectomy radiation to the axilla is less than one percent in the region of Andhra Pradesh.
Keywords: axillary radiation; breast cancer; retrospective study
Full Text
Introduction
MNJ Institute of Oncology Regional Cancer Center (MNJIO & RCC) is one of India’s oldest cancer centers, established in 1953 as Radium Institute. The institute started its treatment with CO 60 machines and radium needles for interstitial application. It is presently equipped with state of art Linear Accelerators. However this study dates back to those days when manual planning and 2D simulator (Ximatron) based planning was used in the planning of adjuvant breast radiation. The policy followed by the institution in the adjuvant radiation of breast cancer was developed from the standard texts available at that time [1-3]. The concept of a posterior axillary boost was not part of the institutional policy and hence was not applied. All patients received the standard 3 field technique. The retrospective study included patients treated from 1995 to 2008. The patients who attended OPD were followed up from May 2007 to December 2012 as part of this study.
Inclusion and exclusion criteria: 1) Only patients who received radiation to the chest wall and drainage areas were included. 2) Patients who had undergone either MRM or BCS with confirmatory postoperative histopathological reports were included. 3) All patients should have undergone adjuvant chemotherapy. 4) All Patients should have received an axillary field as part of radiation. No posterior axillary boost should have been received by the patient. 5) The patients who received only medial and lateral tangents were excluded. To study the axillary recurrences after calculation of radiation dose delivered to the mid axillary plane in breast cancer patients receiving adjuvant radiation.
Materials and methods
Any patient who came to the Outpatient Department (OPD) of MNJIO & RCC for follow up and who satisfied the inclusion criteria were included. There was only one male patient enrolled. All these patients had completed their surgery, chemotherapy and radiation and were attending follow up clinics. The radiation was planned either manually using half beam blocks on the treatment machine itself or on the 2D simulator. Standard tangent angles were used when planning was done manually. A junction gap of 5-7mm was maintained between the supraclavicular field and tangents as part of junction matching (½ L1 d1/ssd1 + L2 d2/ssd2). The dose of the tangents was prescribed to the mid plane. The dose to the supraclavicular field prescribed to 3cm depth irrespective of the axillary separation. An extended supraclavicular field was planned that extended from the midline up to the insertion of deltoid. No posterior field or boost was planned. None of the patients received an IMC field. The junction was placed at the level of 2nd intercostal space and high tangents were not used. The axillary separation was measured at the level of the 2nd intercostal space. The axillary separation of each patient was calculated at the first OPD visit.
The dose received to the midaxillary plane was extrapolated based on the ratio of the percentage depth dose at the prescription point i.e., the normalization point of the prescription (3cm) to the percentage depth dose at the axillary mid plane. The equivalent square of the supraclavicular field was first calculated via the equation 2ab/a+b. The dose to the D1/2 was then calculated empirically based on the ratio of the PDD at 3cm Vs the PDD at the D1/2. The PDD’s were determined during machine commissioning.
Patients who attended the OPD from May 2007 to Dec 2012 were followed up for recurrences. As the medical records were destroyed in a natural calamity, the follow up had to be stopped. Among the 208 patients followed up, 60 were treated on the linear accelerator and 148 on the CO60 machine. The cobalt was operational since 50 years and the linear accelerator was installed in 2008 and hence there was a discrepancy in numbers. However it did not affect the results of the study. All the patients had undergone surgery and chemotherapy. All the patients had received chemotherapy with AC, FAC, FEC, AC-T or CMF regimens. Hormonal therapy was given to those with positive ER PR status. Majority underwent an MRM as the facilities to deliver a boost to the lumpectomy cavity were unavailable.
Results
The mean duration of follow up in the cobalt arm was 53.66 months with a range of 6-162 months. The mean duration of follow up in the Linear Accelerator arm was 14.03 months ranging from 6 to 43 months. The mean dose prescribed to the supraclavicular fossa in cobalt arm was 4831.62 cGy (range of 44Gy to 51Gy) and 4872.08cGY (range 45Gy to 50.4Gy) in the LA arm. The actual mean dose received by the axilla was in the cobalt arm 4058.9 cGy (range 3301cGy to 4705cGy) and 4134.77 cGY (range 3597cGy to 4824cGy) in the LA arm, none of which were statistically different (Table 1). The mean treatment depth (axillary mid plane) in the cobalt arm was 5.85cm (range 4 to 8.5 cm) and 6.2cm (range 4.5cm to 8cm) in the LA arm which was also statistically non-significant (Table 1).
Table 1: Comparison of cobalt 60 and LA patients with statistics.
| Parameter |
Cobalt 60 |
Linear Accelerator |
2 tailed t test |
| Mean Age |
49.09 |
48.31 yrs |
p= 0.6193 (CI 95%) |
| Mean follow up months |
53.66 |
14.03 |
P= 0.0314 (CI 95%) |
| Mean prescription D1/2 |
4831.62cGy |
4925.66cGy |
P = 0.0044 (CI 95%) |
| Mean received by D1/2 |
4058.9cGy |
4212.73cGy |
P= 0.0012 (CI 95%) |
| Mean separation D1/2 |
5.89cm. |
6.2cm |
P=0.0314 (CI 95%) |
| Positive margins |
8 (5.4%) |
4 (6.6%) |
P=0.7253 (CI 95%) |
| Close surgical margins |
6 (4.0%). |
2 (3.3%) |
P=0.9934 (CI 95%) |
| Triple negative |
12 (8.1%) |
14 (23.3%) |
P=0.025 (CI 95%) |
| Nodal status N0 |
56 (37.8%) |
27 (45%) |
Chi-Square value |
| Nodal status N1 |
49 (33.1%) |
15 (25%) |
0.913 P<0.50 (CI 95%) |
| Nodal status N2 |
20 (13.5%) |
6 (10%) |
1 degree of freedom |
| Nodal status N3 |
N3 in 8 (5.4%). |
3 (5%) |
|
| Nodal status NA |
15 (10.1%) |
9 (15%) |
|
| Inadequate dissection |
57 (38.5%) |
18 (30%) |
Chi – square value |
| MRM |
142 (95.9%) |
58 (96.6%) |
5.58p = 0.1352 (CI 95%) |
| BCS |
6 (4.1%) |
2 (3.4%) |
|
| Adjuvant hormonal |
115 (77.7%) |
25 (41.6%) |
3 degree of freedom |
|
Abbreviations: NA: not available, MRM: modified radical mastectomy, BCS: breast conservative surgery.
|
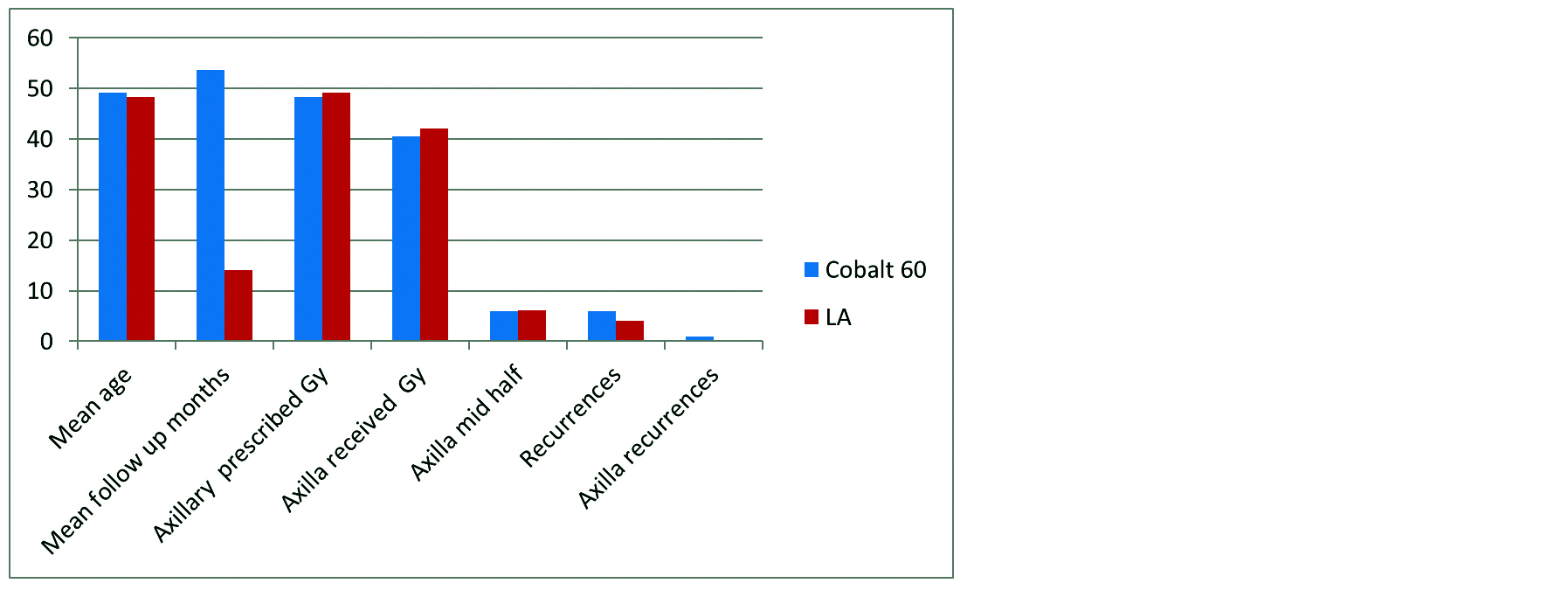
Figure 1: Cobalt and LA.
Recurrences
In the cobalt arm there were 6 recurrences (4.0%) which includes one malignant pleural effusion, one contra lateral breast, one ipsilateral chest wall, one ipsilateral axilla, one liver and one bone metastasis.
In the linear accelerator arm there were 4 recurrences (6.6%) which includes one malignant pleural effusion, one contra lateral breast, one ipsilateral chest wall and one bone + supraclavicular node metastasis. The incidence of ipsilateral axillary recurrence is 0.67% in the cobalt arm and 0.48% in the entire cohort. The single recurrence occurred in a patient treated in a private hospital with TxNxMo disease with positive surgical margins with 3×4×3 cm tumor with inadequate dissection of 3 nodes and who received 4187cGy to mid axillary plane. The overall incidence of recurrence in both arms is statistically non-significant.
Statistical analysis
The goal of the study was to observe the axillary outcome. However in order to render both groups as comparable, a 2 tailed t test was used to compare the variable parameters. The other parameters were compared using the Chi-Square test with 1 to 3 degrees of freedom.
Discussion
This observational study was conducted as part of the routine follow up clinics. Hence it represented a heterogeneous population of post-mastectomy breast reconstruction (PMBR). Among the group were observed high risk factors like triple negativity (8.1% and 23.3%) N3 nodal status (5.3% and 5%), positive surgical margins (5.4% and 6.6%), all of which are high risk factors for loco-regional and systemic recurrence. Post-mastectomy radiotherapy (PMRT) was introduced in the 1970’s and has shown both improved loco regional control as well as overall survival in several studies till date [4-6]. The present indication for PMRT is tumor size >5cm and/or patients who have four or more involved axillary lymph nodes [7, 8]. There is also data reflecting a positive impact of PMRT on overall survival in patients having 1-3 positive axillary lymph nodes with T1-T2 primary disease provided proper technique is exercised [9, 10].
The axillary and supraclavicular nodes are the draining nodes for the breast and a dose of 45 – 50Gy has been recommended in the adjuvant setting. However, some patients may receive less than the recommended dose of radiation depending upon the technique used for planning external beam radiation. In this study many patients had an inadequate axillary dissection (38.5% and 30%). Many had unknown axillary status too (10.1% and 15%). Sentinel node biopsy was not practiced then. The incidence of lymphedema increases when axillary radiotherapy follows axillary clearance and may be avoided [11].
The low rate of axillary recurrence in this study cannot be explained by complete/incomplete axillary dissection or adjuvant chemotherapy. The present axillary recurrence of 0.04% was derived from the follow up clinic and may not reflect the true institutional axillary recurrence rate as some patients may be lost to follow up after recurrence. As the axillary recurrence rates were very low and the standard supraclavicular fields delivered inadequate radiation, a smaller field covering only the supraclavicular fossa may be an option to maintain locoregional control whilst controlling locoregional morbidity.
Conclusions
This study was not powered to answer questions on the role of axillary radiation in post-mastectomy irradiation. However given the strength of the sample size and the longer duration of follow up, it can be concluded by the author that the incidence of axillary recurrences in a heterogeneous population receiving locoregional post-mastectomy radiation to the axilla is less than one percent in the region of Andhra Pradesh. However this study also raises two important concerns as the vast majority of the cohort received sub therapeutic doses to that axilla but still did not develop an axillary recurrence (mean 4134.77 cGY and 4236.56cGy). 1. Will the addition of a posterior axillary boost field improve the already low axillary recurrence rates? 2. Will the exclusion of the axilla from the loco-regional field affect the rate of axillary recurrences?. Larger prospective trials addressing the dose to the axilla using modern day treatment planning systems may help address the above issues.
Conflicts of interest
Author declares no conflict of interest.
List of abbreviations
PPD: Percentage Depth Dose, LA: Linear accelerator, cGy: Centigray, PMRT: Post-mastectomy radiatherapy, MRM: Modified radical mastectomy, BCS: Breast conservative surgery, AC: Adriamycin cyclophosphamide, FAC: 5FU adriamycin cyclophosphamide, FEC: 5FU epirubicin cyclophosphamide, AC-T: Adriamycin cyclophosphamide paclitaxel, CMF: Cyclophosphamide methotrexate 5FU, ER PR: Estrogen progestrone receptor status. D1/2 - mid plane.
References
[1] Carlos A Perez, Luther W Brady. Principles and practice of radiation oncology, Lippincott-Raven Philadelphia. 1998.
[2] Gilbert H Fletcher. Textbook of radiotherapy, Lea & Febiger, Philadelphia. 1978.
[3] James D Cox. Moss’ Radiation Oncology, Mosby, Philadelphia. 1989.
[4] Ragaz J, Jackson SM, Le N, Plenderleith IH, Spinelli JJ, et al. Adjuvant radiotherapy and chemotherapy in node positive premenopausal women with breast cancer. N Engl J Med. 1997; 337(14):956-962.
[5] Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, et al. Postoperative radiotherapy in high-risk postmenopausal breast cancer patients given adjuvant Tamoxifen: Danish Breast Cancer Cooperative Group DBCG82c randomised trial. Lancet. 1999; 353(9165): 1641–1648.
[6] Whelan TJ, Julian J, Wright J, Jadad AR, Levine ML. Does locoregional radiotherapy improve survival in breast cancer? A meta-analysis. J Clin Oncol. 2000; 18(6):1220–1229.
[7] Recht A, Edge SB, Solin LJ, Robinson DS, Estabrook A, et al. Postmastectomy radiotherapy: Clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001; 19(5):1539–1569.
[8] Harris JR, Halpin-Murphy P, McNeese M, Mendenhall NP, Morrow M, et al. Consensus statement on postmastectomy radiation therapy. Int J Radiat Oncol Biol Phys. 1999; 44(5):989–990.
[9] Ragaz J, Olivotto IA, Spinelli JJ, Phillips N, Jackson SM, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005; 97(2):116–126.
[10] Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b and c randomized trials. Radiother Oncol. 2007; 82(3):247–253.
[11] Novak J, Besic N, Dzodic R, Gazic B, Vogrin A. Pre-operative and intra-operative detection of axillary lymph node metastases in 108 patients with invasive lobular breast cancer undergoing mastectomy. BMC Cancer. 2018; 18(1):137.