Full Text
Introduction
Enterocutaneous fistulae are abnormal communications between the gastrointestinal tract and the skin. Although rare, they are associated with considerable morbidity and mortality. Death related to enterocutaneous fistula remains disproportionately high compared with that associated with other surgical conditions. Studies reported over the past 30 years have shown mortality rates of -41 % which rate of 6-33 % in the most recent case series. Sepsis was the leading cause of death in all of these studies. Increased mortality has been shown to be associated with high initial fistula output and the presence of complications.
The management of enterocutaneous fistula continues to present a considerable challenge to surgeons, gastroenterologists and allied professionals, and this has resulted in a variety of different management strategies. Patients have frequently undergone several operative procedures, and their physiological and nutritional reserves are often severely compromised. Management should focus initially on correction of fluid and electrolyte disturbances, aggressive treatment of sepsis and control of fistula output. Nutritional requirements must be addressed and attention paid to skin care and psychological support. Only after these issues have been dealt with adequately, and if the fistula persists after conservative measures, should further surgery be contemplated arginine, omeza-3 fatty acids, antioxidants and nucleotides in critically ill and postoperative patients. These all show reductions in the rate of infectious complications and light of hospital stay in those receiving enteral immunonutrition compared with standard enteral feeds.
Case report
33 years male patient presented with history of alleged RTA on 16 February 2013 around 8:00pm near Kurnool. When he riding in a two wheeler, hit by a lorry, patient had sustained injuries to hip, could not able to stand or walk after RTA. Patient admitted in local hospital in Kurnool. Treated conservatively CT scan of whole abdomen plain and I.V contrast was done on 17 February 2013. Showed of whole abdomen plain and contrast, Gas distended ascending and transverse colon, intra muscular collection along right lateral abdominal wall, fracture bilateral iliac bones, multiple fractures noted in bilateral acetabulam. During conservative management patient developed abscess over right thigh (Figure 1). It was not healing on regular dressing. For which patient referred to KIMS hospital on 22 March 2013. On examination patient Afebrile, vitals – stable, local examination shortening of right lower limb swelling and redness over anterolateral aspect of thigh and tenderness present. No other comorbidities.
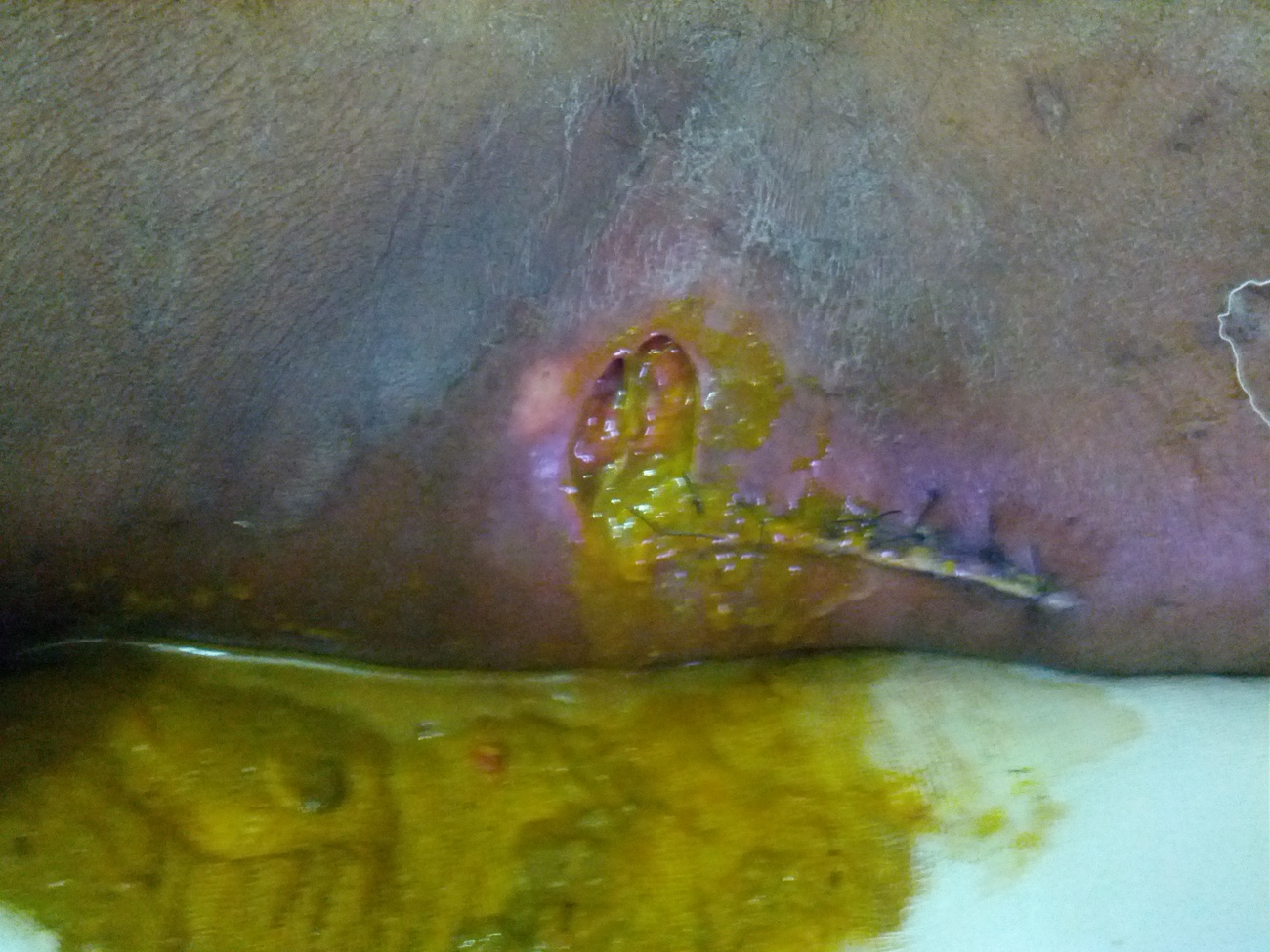
Figure 1: Fistula track opening.
Results and discussion
Patient was evaluated and found to have pelvic fracture with antero-lateral right thigh abscess. Incision and drainage with debridement done on 22 March 2013. Procedure was uneventful. Post procedure, daily dressing done. Patient developed discharge of feculent material from the wound, for which surgical gastroenterology consultation was taken. CECT abdomen showed perforated distal ileal loop with leak of oral contrast and air into lateroconel fascia, extra peritoneal space and extension of bowel contents into right lower anterior abdominal wall and upper 1/3rd of antero-lateral aspect of right thigh, fluid collection extending into right iliacus muscle following fractures are seen in CT (Figure 2A, 2B). Comminuted fracture of right ileum, acetabulum with central dislocation of femur. Comminuted fracture of left acetabulum.
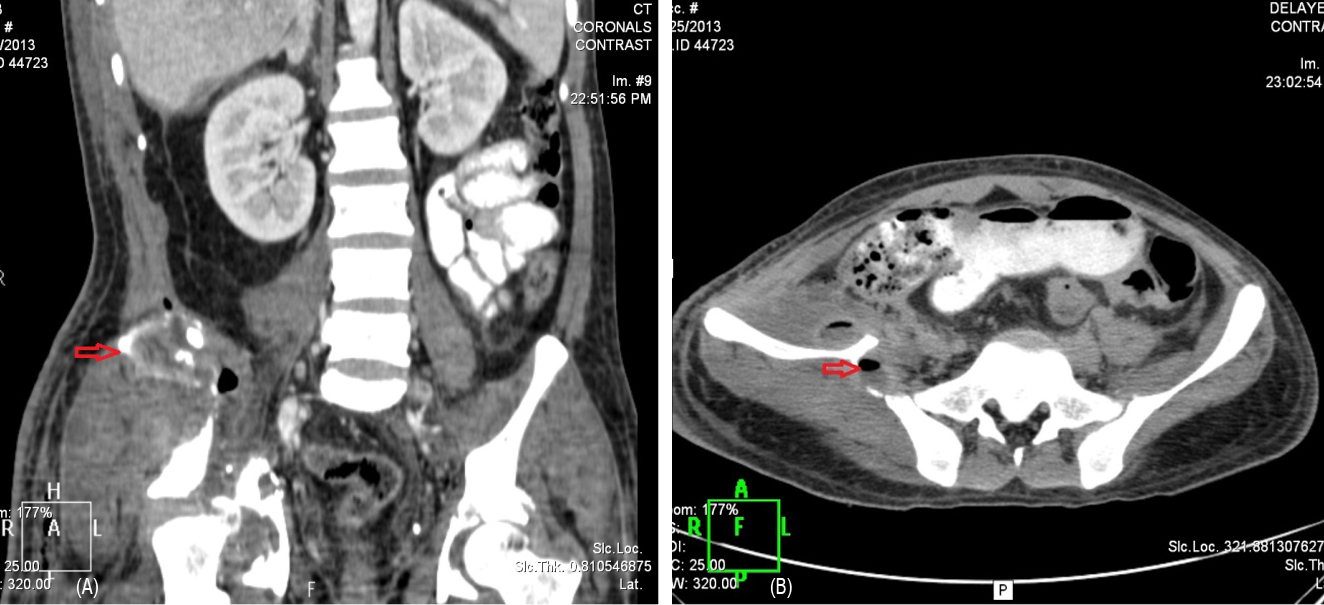
Figure 2: (A) Contrast leak into thigh, (B) Right pelvic fracture.
Over the days, patient developed fever, raised total WBC count, tachycardia. Case discussed in the KIMS multidisciplinary meet, which preferred surgical repair over conservative treatment. Patient taken up for surgery, after through pre-op work up and evaluation.
Surgery on 28/03/2013: "Exploratory laparotomy and right hemicolectomy with closure of colonic stump and end ileostomy + peritoneal lavage; followed by irrigation and washout of right thigh abscess"
Intra operative findings
1) Dense omental, intestinal adhesions to anterior, lateral abdominal well. 2) Purulent peritoneal fluid. 3) Dense, fragile adhesions; making anatomy of small and large intestines distorted; formed a mass like structure (Cocoon) in the pelvis. 4) Bowel anatomy defined after careful adhesiolysis. 5) Complete transection of bowel seen near terminal - ileum with adjacent unhealthy bowel. Hence, right hemicolectomy was done. 6) Purulent collection seen in pelvis tracking from right iliac fossa into antero-lateral aspect of right thigh wound; forming a fistulous tract (Figures 3A and 3B). Pus sent for c/s study. 7) Bilateral ureters, iliac vessels, urinary bladder identified and well preserved. 8) Thorough peritoneal lavage followed by irrigation and washout of right thigh abscess was done.
Post op, patient kept in SICU, put on higher IV antibiotics, and subsequently shifted to the ward once his general condition improved. Daily wound dressing done. With regular physiotherapy, supportive care patient improved, tolerated oral feeds, and he could now able to walk with support. Patient is being discharged in stable condition. After 4 months, Gastrograffin (Figure 4) was normal then posted for end ileostomy closure (Figure 5). Underwent side to side ileo-colic anastomosis was done and managed conservatively recovered well (Figure 6).
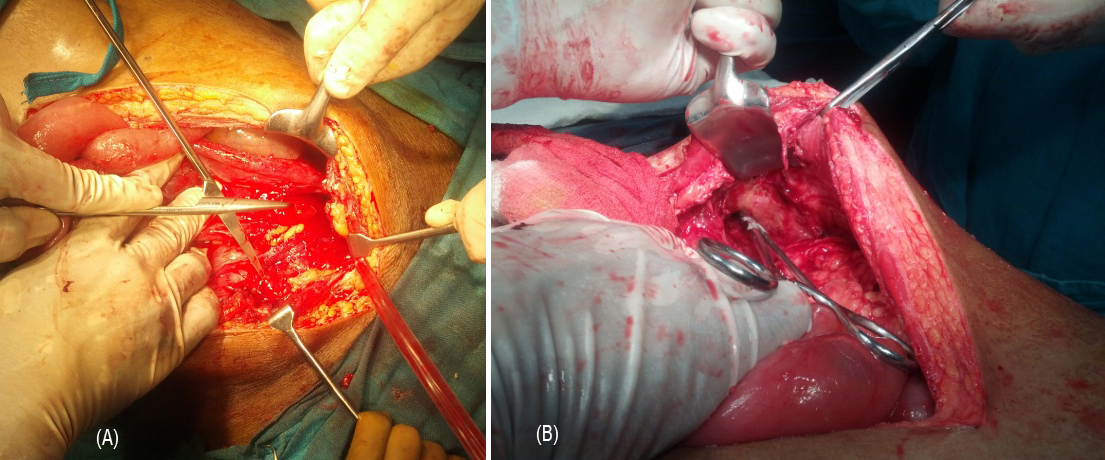
Figure 3: (A) Internal fistula opening, (B) Fistula track through thigh.
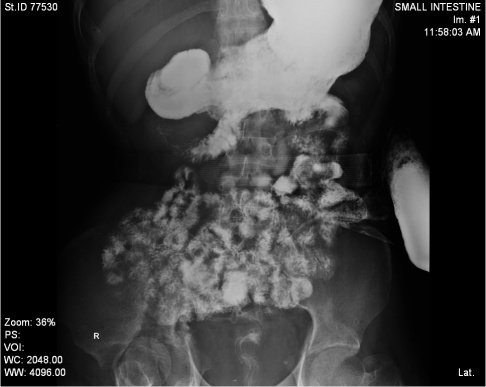
Figure 4: Gastrograffin study.
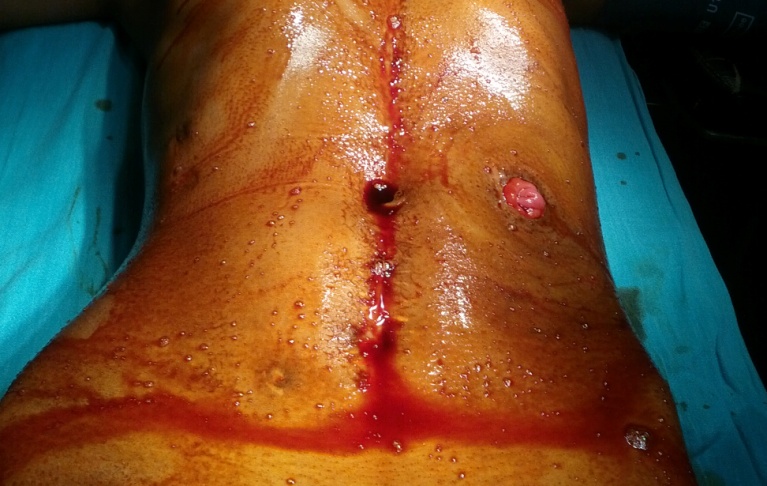
Figure 5: End ileostomy.
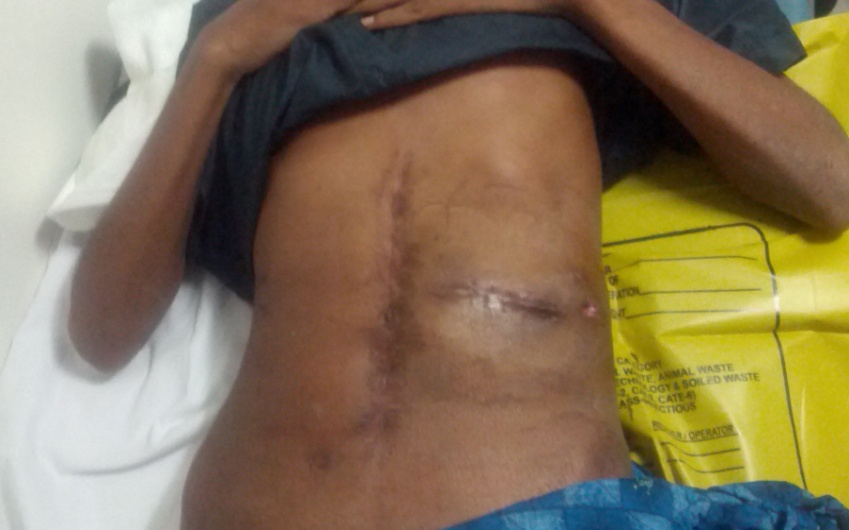
Figure 6: The post-operative scars.
Discussion
Epidemiology and risk factors for ECF in trauma
ECF is an uncommon and not well studied posttraumatic complication. The occurrence of posttraumatic ECF, however, is known to be associated with considerable morbidity. Although the true incidence of this adverse event is unknown, one large study of 2373 patients requiring trauma laparotomy conducted by Teixeira and colleagues identified ECF development in 1.5% [1]. This group found that the development of posttraumatic ECF was associated with significant increase in intensive care unity (ICU) length of stay (28.530.5 vs 7.69.3 days, p¼0.004), hospital length of stay (82.1100.8 vs 16.217.3 days, p<0.001) and mean hospital charges ($539,309 vs $126,996, p<0.001).
Since the introduction of damage control principles for abdominal trauma, open abdominal management has become an increasingly common component of trauma care. The open abdomen may represent particular increased risk for the development of ECF. Several groups have examined this topic, identifying an ECF rate of between 4.5% and 25% following open abdominal management [2-4].
The risk factors for the development of post traumatic ECF are likely multiple. In the aforementioned examination by Teixeira and colleagues, 89% of patients who developed an ECF had an antecedent hollow viscus injury repair; with 56% having multiple hollow viscus injuries [1]. The findings of this group also suggested that location of the viscus injury may prove important, with the majority of ECF in these patients arising from colonic sources. The method of anastomosis following resection of injured bowel has also not proven to play a significant role in the occurrence of ECF after trauma.
In study of 297 patients with penetrating colon injuries conducted by Demetriades and the members of an American Association for the Surgery of Trauma prospective multicenter study group, the investigators found that the choice of stapled or hand-sewn anastomosis did not affect the incidence of anastomotic complications,including leak or fistula development [5].
In a separate report from the same group, they found that the use of colonic diversion over primary anastomosis following penetrating colonic injury also failed to prove protective in avoiding abdominal septic complications, including abscess and fistula [6].
Treatment of ECF in traumatically injured patients
The effective management of ECF is a considerable challenge in posttraumatic care. ECF are associated with a mortality of 36 to 64%, which is markedly higher than current outcomes with more traditional ECF. Unlike ECF with an intact abdominal wall, which have a spontaneous closure rate of 50 to 80%, ECF require surgical intervention the majority of the time to achieve resolution [7-9].
Key components of management include adequate delivery of nutrition, electrolyte/fluid deficit correction, effective control of sepsis and early surgical intervention when possible. For complex or recurrent fistulas, several non-operative approaches designed to provide for control of fistula output have been proposed. The decision of enteral versus parenteral delivery systems, however, remains controversial and is largely dictated by the nature of the fistula and the nutritional status of the patient. Early reports by Deitel and Thomas suggested that patients receiving total parenteral nutrition (TPN) might have twice the ECF closure rate with half of the mortality [10-11].
On the negative side, the delivery of TPN through central venous catheters is associated with an appreciable rate of bacteremia and line sepsis.
In one study conducted by Wang and colleagues, they obtained positive blood cultures from 24.6% of 88 catheters utilized to deliver TPN to patients undergoing non operative management of enteric fistulas [12]. Enteral nutrition has also been advocated as a potential means of support for patients with ECF, with recognized benefits including the protection of mucosal integrity and decreased cost relative to TPN. For proximal fistulas, the intubation of the fistula itself has been utilized effectively for delivery of enteral nutrition.
To date, however, there have been no well-designed studies comparing effectively the impact of TPN versus enteral nutrition for the management of ECF after trauma or in the setting of critical illness. The timing and extent of operative intervention for ECF has not been well studied. Early operative approaches have been advocated, particularly in the setting of the open abdomen, where a window of opportunity prior to the onset of the ‘‘frozen abdomen’’may exist. Once the development of dense adhesions has occurred, however, initial nonoperative management with planning for delayed surgical intervention for persistent fistula is advisable. The obliterative peritonitis that develops takes at least 4 months to subside to allow for safe laparotomy and adhesiolysis [13]. In both the acute and chronic settings, recurrence rates after operative treatment of ECF are high. In a retrospective study of 205 patients undergoing operative treatment of ECF from various causes, Lynch et al found that 20.5% developed a recurrence within 3 months of intervention [14]. Multivariate analysis by this group demonstrated that ECF recurrence was more likely after oversewing than when resectional approaches were utilized (36% vs 16%, respectively, p¼0.006). In a subsequent examination of 135 patients undergoing operative repair of ECF, Brenner and colleagues 28 found that recurrence was more common when resection was performed by stapled anastomosis (35%) than when resection and hand-sewn anastomotic approaches were utilized (11%) [15].
Conclusion
Treatment of enterocutaneous fistula should concentrate initially on correction of fluid and electrolyte imbalances, drainage of collections, treatment of sepsis and control of fistula output. Malnutrition is common, and nutritional assessment and provision are essential. Although restriction of enteral intake and ‘bowel rest’ are often advocated, there is no evidence to suggest that such practice results in increased rates of fistula closure, and it may even increase the incidence of complications. Enteral nutrition should be used if possible, although high output small bowel fistula usually require supplemental parenteral nutrition. Operative repair should be preformed when spontaneous closure does not occur, but it should be delayed at least for 3 months.
Conflicts of interest
Authors declare no conflicts of interest.
References
1. Teixeira PG, Inaba K, Dubose J, Salim A, Brown C, et al. Enterocutaneous fistula complicating trauma laparotomy: a major resource burden. Am Surg. 2009; 75(1):30–32.
2. Jamshidi R, Schecter WP. Biological dressings for the management of enteric fistulas in the open abdomen: a preliminary report. Arch Surg. 2007; 142(8):793–796.
3. Tsuei BJ, Skinner JC, Bernard AC, Kearney PA, Boulanger BR. The open peritoneal cavity: etiology correlates with the likelihood of fascial closure. Am Surg. 2004; 70(7):652–656.
4. Miller RS, Morris JA Jr, Diaz JJ Jr, Herring MB, May AK. Complications after 344 damage-control open celiotomies. J Trauma. 2005;59(6):1365–1371, discussion 1371–1374.
5. Demetriades D, Murray JA, Chan LS, et al. Handsewn versus stapled anastomosis in penetrating colon injuries requiring resection: a multicenter study. J Trauma. 2002;52(1):117–121.
6. Demetriades D, Murray JA, Chan L, et al; Committee on Multicenter Clinical Trials. American Association for the Surgery of Trauma. Penetrating colon injuries requiring resection: diversion or primary anastomosis? An AAST prospective multicenter study. J Trauma. 2001;50(5):765–775.
7. Prickett D, Montgomery R, Cheadle WG. External fistulas arising from the digestive tract. South Med J. 1991;84(6): 736–739.
8. Martinez D, Zibari G, Aultman D. The outcome of intestinal fistulae: the Louisiana State University Medical Center—Shreveport experience. Am Surg. 1998;64(3):252–254.
9. Sriussadaporn S, Sriussadaporn S, Kritayakirana K, Pak-art R. Operative management of small bowel fistulae associated with open abdomen. Asian J Surg. 2006;29(1):1–7.
10. Deitel M. Nutritional management of external gastrointestinal fistulas. Can J Surg. 1976;19(6):505–509.
11. Thomas RJ. The response of patients with fistulas of the gastrointestinal tract to parenteral nutrition. Surg Gynecol Obstet. 1981;153(1):77–80
12. Wang GF, Ren JA, Jiang J, Fan CG, Wang XB, Li JS. Catheter-related infection in gastrointestinal fistula patients. World J Gastroenterol. 2004;10(9):1345–1348.
13. Osborn C, Fischer JE. How I do it: Gastrointestinal cutaneous fistulas. J Gastrointest Surg. 2009;13(11):2068–2073.
14. Lynch AC, Delaney CP, Senagore AJ, Connor JT, Remzi FH, Fazio VW. Clinical outcome and factors predictive of recurrence after enterocutaneous fistula surgery. Ann Surg. 2004;240(5):825–831.
15. Brenner M, Clayton JL, Tillou A, Hiatt JR, Cryer HG. Risk factors for recurrence after repair of enterocutaneous fistula. Arch Surg. 2009;144(6):500–505.