Orginal Research
2015
March
Volume : 3
Issue : 1
Relationship between parathyroid hormone and serum creatinine levels in chronic kidney disease patients
Radhika Chowdary D, Rohini, Prasad Reddy
Pdf Page Numbers :- 17-21
Radhika Chowdary D1,*, Rohini1 and Prasad Reddy1
1Department of Biochemistry, Laboratory Services, Krishna Institute of Medical Sciences, Minister Road, Secunderabad-500003, Telangana, India.
*Corresponding author: Dr. D. Radhika Chowdary, MD (Biochemistry), Consultant Biochemist & Chief of Labs, Department Laboratory Medicine, Krishna Institute of Medical Sciences, Minister Road, Secunderabad - 500003, Telangana, India. Email: rchowdary13@gmail.com
Received 16 September 2014; Revised 2 December 2014; Accepted 18 December 2014; Published 23 December 2014
Citation: Radhika Chowdary, Rohini, Prasad Reddy. Relationship between parathyroid hormone and serum creatinine levels in chronic kidney disease patients. J Med Sci Res 2015; 3(1):17-21. DOI: http://dx.doi.org/10.17727/JMSR.2015/3-003
Copyright: © 2015 Radhika Chowdary, et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Chronic kidney disease (CKD) is a chronic and progressive disease characterized by renal dysfunction due to the decrease of the glomerular filtration rate (GFR). Hyperparathyroidism—an elevation of parathyroid hormone (PTH) is a complication of CKD.
Objective of the study: The present study is a retrospective study focussed on the relationship between PTH levels and serum creatinine/ GFR in CKD patients based on OP follow up.
Materials and methods: The present study included 150 subjects who have been diagnosed as CKD and 80 normal subjects at Department of Laboratory services, Krishna Institute of Medical Sciences, Secunderabad, India. The CKD population included both males (75) and females (75) and the normal population included both males (40) and females (40) with an age group between 22 years and 87 years. The measurements of creatinine and PTH are done on the serum in Unicel DXC860i fully automated random access analyser by Modified Jaffe’s method traceable to IDMS. PTH is analysed by CLIA method. The GFR is calcutated by MDRD formula and using creatinine measured in serum.
Results: The main finding in the study was a significant positive correlation between PTH and creatinine in CKD patients when compared to normal patients.We observed a significant rise in the PTH values with an increase in creatinine values and the increase in PTH is statistically higher in females(r=0.121, p<0.001) when compared to males (r=0.557, p<0.0001) in CKD patients. While in normal population the correlation between PTH and creatinine was insignificant, females (r=0.06, p<0.0001) and a significant negative correlation was observed between PTH and creatinine in males (r =-0.22, p<0.0001).
Conclusion: Hyperparathyroidism is one of the earliest manifestations of impaired renal function.Monitoring trends is important for the detection and treatment of Chronic Kidney Disease. In these patients, it is reasonable to base the frequency of PTH monitoring on the presence and magnitude of abnormalities and rate the progression of disease. Progressive increases of PTH should be avoided and marked changes in PTH levels should trigger interventions with relatively simple treatments that have the potential to prevent adverse outcomes.
Keywords: Parathyroid hormone; serum creatinine; chronic kidney disease
Full Text
Chronic renal failure is defined as either kidney damage with glomerular filtration rate less than 60 ml/min for three months or more [1]. This is invariably a progressive process that results in end stage renal disease. In India, the projected number of deaths due to chronic disease was around 5.21 million in 2008 and is expected to rise to 7.63 million in 2020 (66.7% of all deaths) [2]. The serum creatinine concentration is widely interpreted as a measure of the glomerular filtration rate (GFR) and is used as an index of renal function in clinical practice [3]. Detection of early renal dysfunction remains a problem because creatinine levels often do not become abnormal until glomerular filtration rate is severely reduced [4]. The two main causes of chronic kidney disease are diabetes and high blood pressure, which are responsible for up to two-thirds of the cases. Glomerular filtration rate (GFR) is the best test to measure the level of kidney function and determine the stage of kidney disease. Hyperparathyroidism - an elevation of parathyroid hormone (PTH) is a complication of CKD. Patients with mild CKD may be asymptomatic and therefore may not be identified until the pathology of secondary hyperparathyroidism (SHPT) has begun. Secondary hyperparathyroidism describes a complex alteration in bone and mineral metabolism that occurs as a direct result of chronic kidney disease [5].
Pathophysiology of secondary hyperparathyroidism
The parathyroid glands are four pea-sized glands located on the thyroid gland in the neck. The thyroid and parathyroid glands produce distinct hormones with specific functions. The parathyroid glands secrete parathyroid hormone (PTH), a polypeptide that helps maintain the balance of calcium and phosphorous in the body. PTH is involved in the homeostasis of bone metabolism by regulating the level of calcium in the blood, release of calcium from bone, absorption of calcium from the intestine, and excretion of calcium in the urine. Consequently, the levels of calcium and other minerals involved in bone metabolism, such as phosphorus and vitamin D, affect the secretion of PTH by the parathyroid gland [6]. The entire PTH molecule is composed of a sequence of 84 amino acids referred to as the intact hormone (iPTH). Although smaller fragments of this molecule may have unique actions in the body, generally, the iPTH is measured and used to assess bone metabolism and bone disease.
SHPT secondary to CKD is an overproduction of PTH caused by changes that occur in bone and mineral metabolism as a result of decreased kidney function (Figures 1 and 2). The first changes that usually occur with declining kidney function involve the deficiency of activated vitamin D and an increase in phosphorus excretion by the remaining functional nephrons. Both of these changes stimulate an increase in PTH synthesis and secretion.
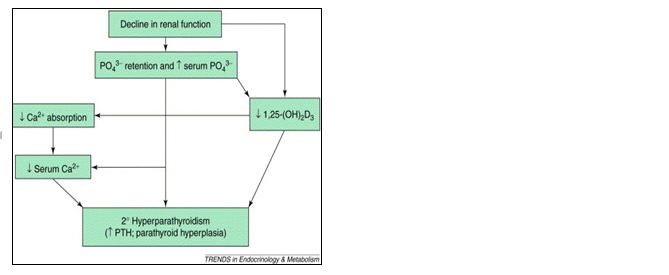
Figure 1: Pathophysiology of secondary hyperparathyroidism.

Figure 2: PHT pathway
Materials and methods
The present study included 150 subjects who have been diagnosed as CKD only on OP follow up. Eighty normal subjects at Department of Laboratory Services, Krishna Institute of Medical Sciences (KIMS), Secunderabad, India. The CKD population included both males (75) and females (75) and the normal population included both males (40) and females (40) with an age group between 22 years and 87 years. The blood samples were randomly collected from patients presenting to the sample collection, Department of Laboratory Services, KIMS Hospital, Secunderabad, using gel vacutainers (yellow) of BD type. After seperation of serum by centrifugation the sample measurements of creatinine and PTH are done in Beckman coulter (Unicel DXC860) using a dedicated reagent. Glomerular filtration rate (GFR) is a calculation of the creatinine clearance. It is calculated from the creatinine, age, body size and sex of the patient. The GFR determines the “stage” of kidney disease. The higher the GFR, the better the kidneys function. The GFR is calcutated by MDRD formula and using creatinine measured by Modified Jaffe’s method traceable to IDMS [7]. Serum intact PTH is analysed by CLIA method [8]. Medical intervention and the status of serum calcium, phosphate, alkaline phosphate, vitamin D, bicorbonate levels are not included in the present study.
Statistical analysis
Data was analyzed using the Graph pad prism. Continuous variables were demonstrated as the mean ± SEM. In order to evaluate the quality and quantity parameters we used the unpaired “t” test. We used the Pearson correlation test for the evaluation of relationships of serum parameters. P values <0.05 were considered significant. Correlation coefficient and linear regression plots were plotted using online Alcula statistical calulators.
Results
The main finding in the study was a significant positive correlation between PTH and creatinine in CKD patients. There was a significant rise in the PTH values with an increase in creatinine values and the increase in PTH is statistically higher in females (r=0.121, p<0.001) when compared to males (r = 0.557, p<0.0001) in CKD patients (Tables 1 and 2, Figures 3 and 4). While in normal Population in females the correlation between PTH and creatinine was insignificant. (r=0.06, p< 0.0001) and a significant negative correlation was observed in males (r = -0.22, p<0.0001) between PTH and creatinine (Tables 3 and 4; Figures 5 and 6).
Table 1: PTH and creatinine in CKD males.
|
|
N
|
Mean
|
StDev
|
SE Mean
|
p Value
|
|
PTH
|
75
|
295.66
|
209.59
|
24.20
|
< 0.0001
|
|
Creatinine
|
75
|
6.98
|
7.04
|
0.81
|
Table 2: PTH and creatinine in CKD females.
|
|
N
|
Mean
|
StDev
|
SE Mean
|
p value
|
|
PTH
|
75
|
338.36
|
287.79
|
33.23
|
< 0.0001
|
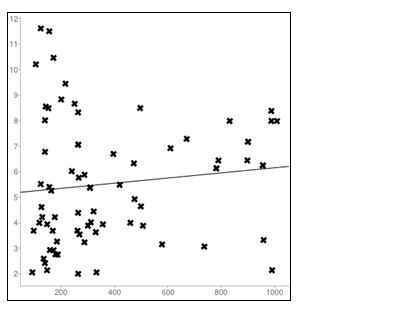
Figure 3: Linear regression plot in CKD Males (r=0.55); X axis= PTH; Y axis=Creatinine.
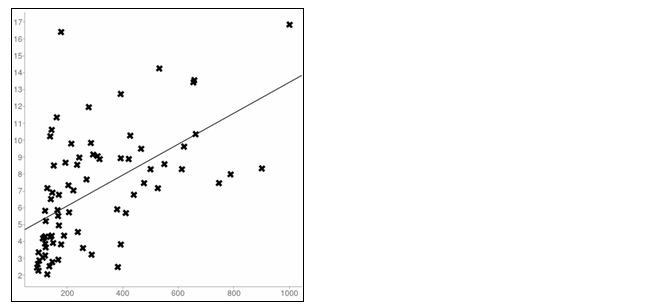
Figure 4: Linear regression plot in CKD Females (r=0.121); X axis= PTH; Y axis= Creatinine.
Table 3: PTH and creatinine in normal males.
|
|
N
|
Mean
|
StDev
|
SE Mean
|
p value
|
|
PTH
|
40
|
42.45
|
15.30
|
2.41
|
< 0.0001
|
|
Creatinine
|
40
|
0.94
|
0.17
|
0.02
|
Table 4: PTH and creatinine in normal females.
|
|
N
|
Mean
|
StDev
|
SE Mean
|
p value
|
|
PTH
|
40
|
44.61
|
21.43
|
3.38
|
< 0.0001
|
|
Creatinine
|
40
|
0.70
|
0.22
|
0.03
|
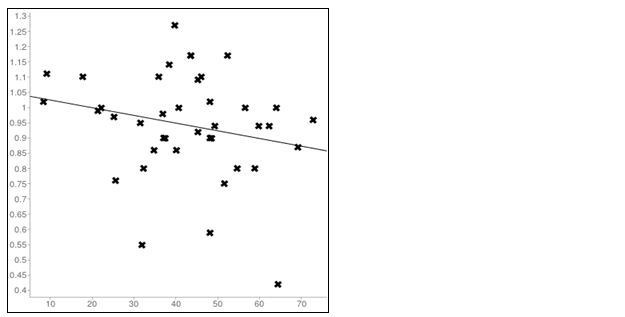
Figure 5: Linear regression plot inNormal Males (r=-0.22); X axis=PTH; Y axis=creatinine.
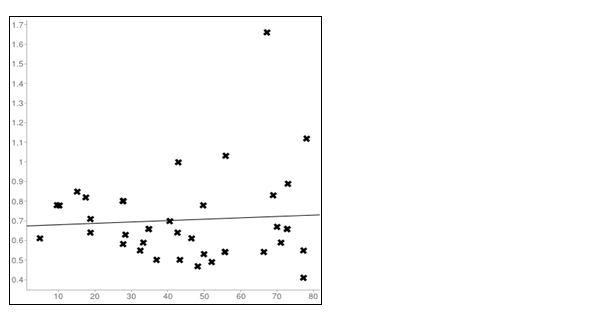
Figure 6: Linear regression plot in Normal Females(r =0.06); X axis= PTH; Y axis= creatinine
Discussion
Under normal physiologic conditions, the concentration of calcium in serum and in cells is tightly controlled. Calcium exists in 3 states in the body; bound to protein, bound to small anions, and in the free (ionized) state. The concentration of serum calcium in the ionized state is regulated by parathyroid hormone (PTH) and 1,25 dihydroxy vitamin D. Circulating calcium is excreted by glomerular filtration and reabsorbed in the proximal tubules. Calcium reabsorption in the proximal tubule is affected by tubular sodium concentration, whereas PTH induces calcium uptake in the distal tubule and the collecting duct. Excess is excreted in the urine and the feces.PTH increases renal tubular reabsorption of calcium. Kidneys play an important role in maintaining bone health. Bones need a balanced level of calcium and phosphorus in the blood to stay strong. When kidney function declines, phosphorus may build up in the blood, causing calcium levels in the blood to become low. This leads to the stimulation of the parathyroid gland releasing parathyroid hormone (PTH) into the blood. The purpose of PTH is to keep calcium levels normal in the blood. When calcium levels are low, PTH moves calcium out of the bones. Over time, as more calcium leaves the bones, the bones become brittle. Hyperparathyroidism is one of the earliest manifestations of impaired renal function [9]. SHPT is an insidious disease that develops early in the course of CKD and increases in severity as the glomerular filtration rate deteriorates. Parathyroid hormone levels begin to rise when creatinine clearance falls below 60 mL per minute (1 mL per second) [1]. In the present study we have found a significant positive association between serum PTH and creatinine in both genders. Increase in PTH is significantly higher in females than in males. While in normal Population in females the correlation between PTH and creatinine was insignificant and a significant negative correlation was observed in males between PTH and creatinine.
Conclusion
Hyperparathyroidism is one of the earliest manifestations of impaired renal function. Monitoring trends in PTH and Creatinine levels is important for the detection and treatment of chronic kidney disease. In these patients, it is reasonable to base the frequency of PTH monitoring on the presence and magnitude of abnormalities and rate the progression of disease. Early identification and active management of patients with renal impairment in primary care can improve outcomes. Marked changes in PTH and creatinine levels should trigger interventions with relatively simple treatments that have the potential to prevent adverse outcomes.
Acknowledgements
We would like to acknowledge KIMS hospital for giving us the opportunity to carry out the study.
Conflict of interest
The authors declare no conflict of interest.
References
1. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002; 39(2 Suppl 1):S1–266.
2. Global status report on noncommunicable diseases (2010). Accessed on September 2012 from http://www.who.int/nmh/publications/ncd_report_full_en.pdf
3. Jafar TH, Schmid CH, Levey AS. Serum creatinine as marker of kidney function in South Asians: a study of reduced GFR in adults in Pakistan. J Am Soc Nephrol. 2005; 16(5):1413–1419.
4. Walser M. Assessment of renal function and progression of disease. Curr Opin Nephrol Hypertens. 1994; 3(5):564–567.
5. Nikodimopoulou M and Liakos S. Secondary hyperparathyroidism and target organs in chronic kidney disease. Hippokratia. 2011; 15(Suppl 1): 33–38..
6. Slatopolsky E, Berkoben M, Kelber J, Brown A, Delmez J. Effects of calcitriol and non-calcemic vitamin D analogs on secondary hyperparathyroidism. Kidney Int Suppl. 1992; 38:S43–49.
7. Product insert of Beckman coulter synchron systems chemistry information sheet- Creatinine Modular Kit Reorder # 472525 © Copyright 2005 Beckman Coulter, Inc.
8. Product insert of Beckman coulter Access Immunoassay systems – PTH Intact REF A16972 © Copyright 2005 Beckman Coulter, Inc.
9. Martinez I, Saracho R, Montenegro J, Llach F. The importance of dietary calcium and phosphorus in the secondary hyperparathyroidism of patients with early renal failure. Am J Kidney Dis. 1997; 29:496–502.