Original Research
2018
June
Volume : 6
Issue : 2
Prevalence and antibiogram of Pseudomonas aeruginosa isolated from various clinical samples in a tertiary care ICU setting
Sukrutha Gopal Reddy, Anil Kumar Bilolikar, Prasanna Lakshmi Kakarla, Udayasree B
Pdf Page Numbers :- 44-48
Sukrutha Gopal Reddy1,*, Anil Kumar Bilolikar1, Prasanna Lakshmi Kakarla1 and Udayasree B1
1Department of Microbiology, Krishna Institute of Medical Sciences, Minister Road, Secunderabad-500003, Telangana, India
*Corresponding author: Dr. Sukrutha Gopal Reddy, Consultant Microbiologist, Krishna Institute of Medical Sciences Ltd, Minister Road, Secunderabad – 500003, Telangana State, India. Mobile: 9177111719; Email: sukruthagopalreddy@gmail.com
Received 31 January 2018; Revised 09 March 2018; Accepted 17 March 2018; Published 23 March 2018
Citation: Reddy SG, Bilolikar AK, Kakarla PL, Udayasree B. Prevalence and antibiogram of Pseudomonas aeruginosa isolated from various clinical samples in a tertiary care ICU setting. J Med Sci Res. 2018; 6(2):44-48. DOI: http://dx.doi.org/10.17727/JMSR.2018/6-8
Copyright: © 2018 Reddy SG et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Pseudomonas aeruginosa is a leading cause of nosocomial infections. Increased resistance in this organism continues to cause a significant threat to patient care because of limited therapeutic options. Knowledge of the prevalence of P. aeruginosa in various infections and its antimicrobial susceptibility pattern is one of the salient attributes for selection of appropriate therapy.
Objective: To determine the prevalence and antibiotic resistance pattern of P. aeruginosa from various clinical samples collected from different ICUs.
Methodology: From 10,332 clinical specimen received over a period of one year, a total of 267 P. aeruginosa isolates were identified and minimum inhibitory concentrations for various antibiotics was found out with help of automated method VITEK 2 (bioMerieux).
Results: A total of 267 (2.58%) P. aeruginosa isolates were identified from various clinical specimens. Maximum number 49 (18.35%) of isolates were recovered from patients in 61-70 years age group. The sensitivity pattern of the isolates is as follows - colistin (80.14%), amikacin (52.05%), ciprofloxacin (47.19%), gentamicin (46.55%), cefepime (44.56%), imipenem (42.69%), cefoperazone/ sulbactam (42.69%), levofloxacin (41.57%), meropenem (39.70%), ceftazidime (38.20%), piperacillin/ tazobactam (31.08%), aztreonam (25.46%) and Ticarcillin/ clavulanic acid (22.47%).
Conclusion: The prevalence and sensitivity of P. aeruginosa varies between communities, hospitals and different patient populations. It is therefore, important to institute a system of surveillance in a hospital so that clinicians have access to recent data on prevalence and antimicrobial resistance which helps in making clinical judgement in therapy.
Keywords: antibiogram; Pseudomonas aeruginosa; ICU setting
Full Text
Introduction
Pseudomonas aeruginosa infection may cause life threatening conditions and mostly it is a hospital acquired infection. P. aeruginosa has the ability to survive on a wide variety of physical conditions, so this organism can live and develop in hospital settings. It is ranked among top five hospitals acquired infections. P. aeruginosa can contaminate many items; the floors, sinks in hospitals, bed rails and also isolated from the hands of staff [1]. Additionally injured patients, soaps, antiseptics; respiratory equipment's and physiotherapy pools are also important source of infections [2]. It can infect almost any external site or organ, and therefore, can be isolated from various body fluids such as sputum, urine, wounds, eye or ear swabs and from blood [3]. It is also a common infection among immunocompromised patients.
Infections caused by P. aeruginosa are frequently life-threatening and difficult to treat as it exhibits intrinsically high resistance to many antimicrobials [4]. Resistance to multiple drugs is usually the result of combination of different mechanisms in a single isolate. There is variety of mechanisms involved in the resistance of P. aeruginosa. Over expression of efflux pumps, acquisition of Extended-spectrum β-lactamases (ESBLs) and Metallo-β-lactamases (MBLs), target site or outer membrane modification are predominant. Production of multiple β-lactamases by P. aeruginosa has led to tremendous therapeutic consequences and posed clinical challenges. ESBLs mediate resistance to extended spectrum cephalosporins such as cefotaxime, ceftriaxone and ceftazidime.
The carbapenems and β-lactam and β-lactamase inhibitor combination such as piperacillin/ tazobactam are the drugs active against ESBL producing P. aeruginosa. However, resistance to these drugs has also been increasing worldwide. The present study aimed to determine the prevalence of P. aeruginosa from clinical specimens and their antibiogram from intensive care units among patients admitted to Krishna Institute of Medical Sciences, Secunderabad.
Material and methods
The present study was conducted in the bacteriology laboratory in the Department of Microbiology at Krishna Institute of Medical Sciences, Secunderabad. All samples received from various ICUs from January 2017 to December 2017 were processed for isolation and identification of bacteria according to standard microbiological techniques.
A total of 10,332 clinical samples which included blood, endotracheal secretions, sputum, bronchial wash, cerebrospinal fluid (CSF) and other fluids, pus and urine were collected, from different ICUs. Specimens were processed on blood agar, MacConkey agar and CLED depending on sample requirement. Bacterial colonies were identified by VITEK 2 compact (bioMerieux) automated system and antimicrobial susceptibility testing was done with the same to detect MIC. Antimicrobials used in the panel were amikacin (An), gentamicin (G), ciprofloxacin (Cip), levofloxacin (LEV), ceftazidime (CAZ), cefepime (SFP), imipenem (IPM), meropenem (MEM), doripenem (DOR), colistin (CS), Ticarcillin/ clavulanic acid (TCC), piperacillin/ tazobactam (TZP), cefoperazone/ sulbactam (CFS) and aztreonam (AT). Interpretation of results was done as per CLSI guidelines. All 267 P. aeruginosa isolated during study period were included for data analysis in the study.
Results
A total of 10,332 samples were sent to laboratory for culture and sensitivity from different ICUs. Maximum isolation of P. aeruginosa in number was from ET secretions (127), followed by pus (32). Maximum number 49 (18.35%) of isolates were recovered from patients between 61-70 years. Age group wise distribution of the isolates is shown in Table 1. Isolation of P. aeruginosa is higher in males compared to females. Table 2 shows that frequency of isolation of P. aeruginosa from various clinical samples.
Table 1: Age and sex wise distribution of P. aeruginosa isolates.
|
Age in years
|
Female (%)
|
Male (%)
|
Total (%)
|
|
< 1
|
5
|
22
|
27 (10.11)
|
|
1-10
|
2
|
9
|
11 (4.11)
|
|
11-20
|
1
|
5
|
6 (2.24)
|
|
21-30
|
5
|
23
|
28 (10.48)
|
|
31-40
|
6
|
13
|
19 (7.11)
|
|
41-50
|
7
|
38
|
45 (16.85)
|
|
51-60
|
4
|
37
|
41 (15.35)
|
|
61-70
|
6
|
43
|
49 (18.35)
|
|
71-80
|
7
|
28
|
35 (13.10)
|
|
81-90
|
0
|
6
|
6 (2.24)
|
|
Total (%)
|
43 (16.10)
|
224 (83.89)
|
267 (99.99)
|
The frequency of isolation of P. aeruginosa from various clinical samples from different ICUs was 2.58%.
Table 2: Frequency of isolation of P. aeruginosa from various clinical samples.
|
S. No
|
Clinical sample
|
Total number of samples cultured
|
Number of Pseudomonas aeruginosa isolated
|
Frequency of isolation (%)
|
|
1.
|
Blood
|
6335
|
23
|
8.61
|
|
2.
|
ET secretions
|
1110
|
127
|
47.56
|
|
3.
|
Urine (catheter catch)
|
1090
|
26
|
9.73
|
|
4.
|
Sputum
|
466
|
31
|
11.61
|
|
5.
|
Urine (clean catch)
|
454
|
8
|
2.99
|
|
6.
|
Pus
|
282
|
32
|
11.98
|
|
7.
|
Other fluids
|
199
|
3
|
1.12
|
|
8.
|
CSF
|
184
|
1
|
0.37
|
|
9.
|
Bronchial wash
|
166
|
13
|
4.86
|
|
10.
|
Tissue
|
26
|
3
|
1.12
|
|
|
Total
|
10332
|
267
|
|
Pulmonary samples accounted for majority of the isolates (59.17%) followed by urine (12.72%) and pus (11.98%). There was an isolate from CSF also.
80.14 % P. aeruginosa were sensitive Colistin which is the most sensitive drug followed by Amikacin (52.05%), Ciprofloxacin (47.19%) and Gentamicin (46.55%). Tigecycline (17.60%) was not reported for P. aeruginosa isolated from urine samples. This is shown as graph in Figure 1 and in Table 3.
Table 3: Antibiotic pattern of P. aeruginosa to various antibiotics tested.
|
S. No
|
Antibiotic
|
Sensitive (%)
|
Intermediate (%)
|
Resistant (%)
|
|
1.
|
Amikacin
|
139 (52.05%)
|
5 (1.87%)
|
123 (46.06%)
|
|
2.
|
Aztreonam
|
68 (25.46)
|
51(19.1 0
|
148 (55.43)
|
|
3.
|
Ceftazidime
|
102 (38.2)
|
23 (8.61)
|
142 (53.18)
|
|
4.
|
Ciprofloxacin
|
126 (47.19)
|
10 (3.74)
|
131 (49.06)
|
|
5.
|
Colistin
|
214 (80.14)
|
1 (0.37)
|
52 (19.47)
|
|
6.
|
Doripenem
|
123 (46.06)
|
6 (2.24)
|
138 (51.68)
|
|
7.
|
Gentamicin
|
124 (46.44)
|
11 (4.11)
|
132 (49.43)
|
|
8.
|
Cefepime
|
119 (44.56)
|
7 (2.62)
|
141 (52.8)
|
|
9.
|
Imipenem
|
114 (42.69)
|
1 (0.37)
|
152 (52.92)
|
|
10.
|
Levofloxacin
|
111 (41.57)
|
15 (5.61)
|
141 (52.8)
|
|
11.
|
Meropenem
|
106 (39.7)
|
6 (2.24)
|
155 (58.05)
|
|
12.
|
Cefoperazone/ Sulbactam
|
114 (42.69)
|
40 (14.98)
|
113 (43.32)
|
|
13.
|
Ticarcillin/ Clavulanic acid
|
60 (22.47)
|
48 (17.97)
|
159 (59.55)
|
|
14.
|
Piperacillin/ Tazobactam
|
83 (31.08)
|
20 (7.49)
|
164 (61.42)
|
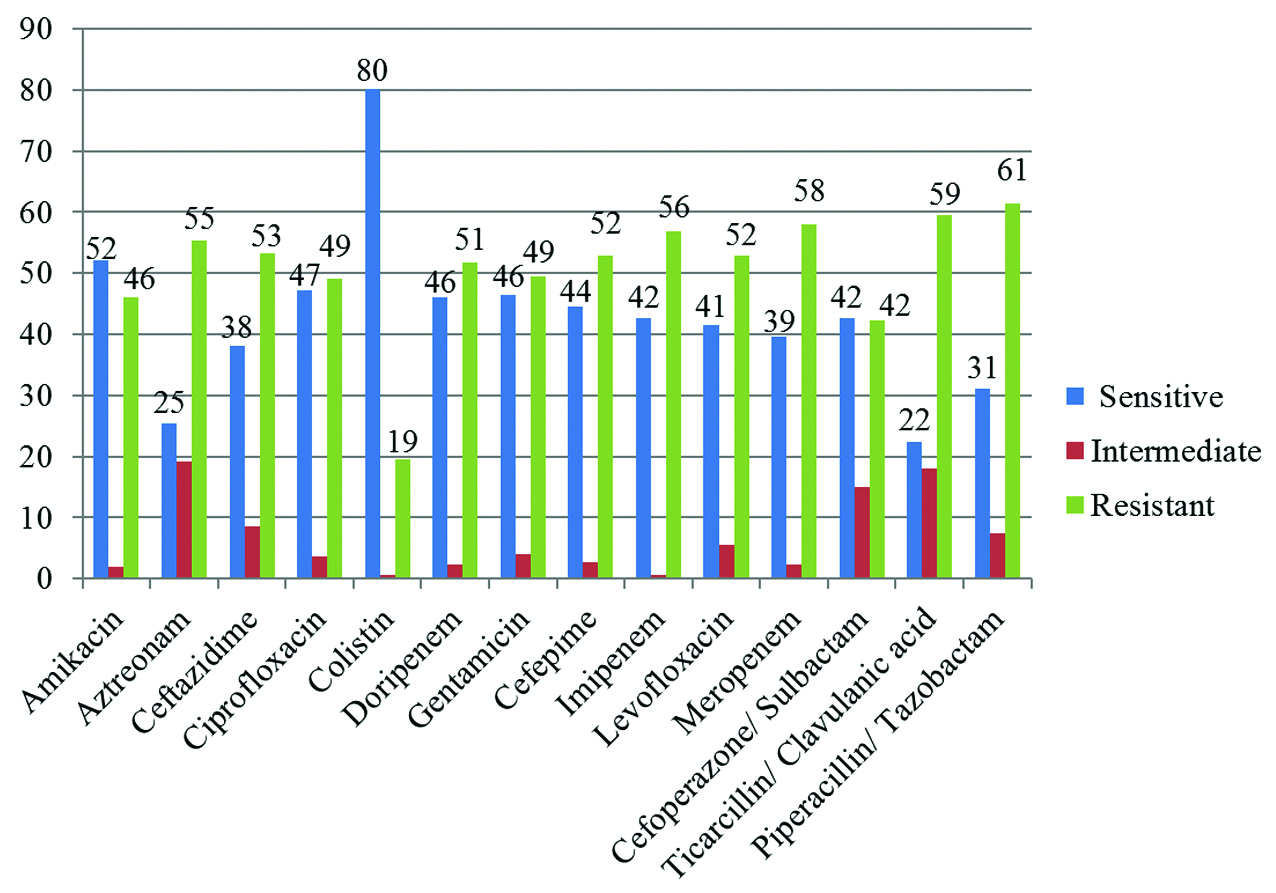
Figure 1: Antibiogram of P. aeruginosa isolated depicted in a column chart.
Discussion
Pseudomonas aeruginosa emerged as important pathogen and is one of the leading causes of morbidity and mortality among hospitalized patients [5]. Hospitalized patients are particularly susceptible to nosocomial infections because the normal skin and mucosal barrier to infection are commonly compromised by use of invasive devices. P. aeruginosa presents a serious therapeutic challenge for treatment of both community acquired and nosocomial infections. Infections caused by P. aeruginosa are notoriously difficult to treat due to its intrinsic ability to resist many classes of antibiotics as well as its ability to acquire resistance [3].
In this study a total of 267 P. aeruginosa strains were isolated and identified from various clinical samples from hospitalized ICU patients. Most of them belong to age group 61-70 (18.35%) followed by 41-50 years (16.85%). Similar age group > 61 was reported to be most affected by Srinivas et al. from Srikakulam [5] and Ahmed et al. from Saudi [6]. This could be due to prolonged hospitalization and other associated co-morbidities in this age group.
Among the isolates, 224 (83.89%) P. aeruginosa were isolated from male patients, similar male predominance was reported by Sharma et al. [7], Anupurba et al. [8], Ahmed et al. [6] and Rashid et al. [9].
In this study P. aeruginosa, pulmonary samples accounted for majority of the isolates (59.17%) followed by urine catheter and clean catch (12.72%) and pus (11.98%). There was an isolate from CSF also. Similar frequency of isolates were observed by Shrestha et al. from Kathmandu [10] other studies reported maximum isolation from pus [11].
The prevalence of P. aeruginosa was 2.58% in this study. However, higher prevalence rates were observed by some other workers; 4.15% [11], 4.6% [12], 5.1% [10] and 9.28% [5]. This can be due to differences in patient population and their hospitalization.
In present study P. aeruginosa exhibited high susceptibility to Colistin (80.14%) followed by Amikacin (52.02%) and Ciprofloxacin (47.19%) and less susceptibility was observed for Aztreonam (25.46%) followed by Ticarcillin / Clavulanic (22.47%), and Tigecycline (17.60%). Sharma et al. [3] reported colistin (95.4%), Amikacin (58%), Aztreonam (47%) and piperacillin (36%) susceptibility and Pawar et al. [13] from western Maharashtra observed susceptibility to colistin (84.84%), Amikacin (37.19%), Ciprofloxacin (25.28%), Ticarcillin – clavulanic (51.99%) and tigecycline (50.55%). Difference in susceptibility can be due to different specimen considered, wide variation in patient population and hospital environment, which can happen even within a single institute.
This study has a few limitations. First, including only hospital acquired isolates of P. aeruginosa of ICU setting. Second, it is essential to conduct a large scale study. Third, molecular typing for resistant genes not done.
Conclusion
The emergence of resistance in P. aeruginosa is an increasing clinical problem, which not only limits future therapeutic choices but is also associated with increased rates of mortality, morbidity and high costs. The prevalence and sensitivity of P. aeruginosa often varies between communities, hospitals in the same community and among different patient populations in the same hospital. It is therefore, important to institute a system of surveillance in a hospital so that clinicians have access to recent data on prevalence and antimicrobial resistance which will help them in making clinical judgement in therapy. Increase in antibacterial resistance in P. aeruginosa is a cause of concern; these are few core actions that will help to fight these deadly infections caused by P. aeruginosa: (i) Preventing infections and preventing the spread of resistance; (ii) Tracking resistant bacteria; (iii) Improving the use of antibiotics; (iv) Promoting the development of new antibiotics and developing new diagnostic tests for resistant bacteria; (v) Strengthen policies, programs, and implementation of infection prevention and control measures.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Hussain MS, Nasir B, Shahid H, Sarwar F, Ejaz A. Prevalence and antibiogram of Pseudomonas aeruginosa isolated from clinical samples at a tertiary care hospital. JSZMC. 2017; 8(2):1185–1188.
[2] Arora D, Jindal N, Kumar R, Romit. Emerging antibiotic resistance in Pseudomonas aeruginosa. Int J Pharm Sci. 2011; 3(2):82–84.
[3] Sharma J, Singh S, Gill AK, Kaur A. Prevalence and antimicrobial susceptibility pattern of Pseudomonas aeruginosa isolated from pus samples in a tertiary care hospital, Bathinda. IJCMR. 2016; 3(12):3481–3483.
[4] Anil C, Shahid RM. Antimicrobial susceptibility patterns of Pseudomonas aeruginosa clinical isolates at a tertiary care hospital in Kathmandu, Nepal. Asian J Pharm Clin Res. 2013; 6(3):235–238.
[5] Srinivas B, Lalitha devi D, Narasingarao B. A prospective study of Pseudomonas aeruginosa and its antibiogram in a teaching hospital of rural setup. JPBMS. 2012; 22(18).
[6] Ahmed OB. Incidence and antibiotic susceptibility pattern of Pseudomonas aeruginosa isolated from inpatients in two tertiary hospitals. Clin Microbiol. 2016; 5:248.
[7] Sharma S, Srivastava P. Resistance of antimicrobial in Pseudomonas aeruginosa. Int J Curr Microbiol App Sci. 2016; 5(3):121–128.
[8] Anupurba S, Bhattacharjee A, Garg A, Sen MR. Antimicrobial susceptibility of Pseudomonas aeruginosa isolated from wound infections. Indian J Dermatol. 2006; 51(4):286–288.
[9] Rashid A, Chowdhury A, Rehman SH, Begum SA, Muazzam N. Infections by Pseudomonas aeruginosa and antibiotic resistance pattern of the isolates from Dhaka Medical College Hospital. Bangladesh J Med Microbial. 2007; 1(2):48–51.
[10] Shrestha S, Amatya R, Adhikari RP. Prevalence and antibiogram of Pseudomonas aeruginosa isolated from clinical specimens in a Teaching Hospital, Kathmandu. Nepal Med Coll J. 2015; 17 (3-4):132-135.
[11] Tadvi J, Javadekar TB, Bhavsar R, Garala N. Prevalence & antibiogram of Pseudomonas aeruginosa at S.S.G. Hospital, Baroda, Gujarat, India. J Res Med Den Sci. 2015; 3(3):204–207.
[12] Sonth SB, Bhurle A, Gokale S. Resistance pattern of Pseudomonas aeruginosa in a tertiary care hospital. Int J Curr Microbiol App Sci. 2015; 4(10):919–923.
[13] Pawar SK, Mane PM, Ravindra V. Pseudomonas aeruginosa and its antibiogram from clinical isolates in a tertiary teaching hospital from western Maharashtra, India. JEBMH. 2014; 7(1):574–581.