Case Report
2018
December
Volume : 6
Issue : 4
Ramipril induced pemphigoid vulgaris
Mustafa Ali Mirza, Suryakanth Jena
Pdf Page Numbers :- 122-124
Mustafa Ali Mirza1,* and Suryakanth Jena1
1Department of Cardiology, Krishna Institute of Medical Sciences, Minister Road, Secunderabad-500003, Telangana, India
*Corresponding author: Mustafa Ali Mirza, Clinical Research Coordinator, Department of Cardiology, Krishna Institute of Medical Sciences, Minister Road, Secunderabad-500003, Telangana, India. Mobile: +91 8523052865; Email: alimirza2395@gmail.com
Received 9 July 2018; Revised 18 August 2018; Accepted 1 September 2018; Published 8 September 2018
Citation: Mirza MA, Jena S. Ramipril induced pemphigoid vulgaris. J Med Sci Res. 2018; 6(4):122-124. DOI: http://dx.doi.org/10.17727/JMSR.2018/6-20
Copyright: © 2018 Mirza MA et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Angiotensin-converting enzyme (ACE) inhibitors are the very frequently used drugs in the treatment of hypertension & heart failure. ACE inhibitors competitively inhibit the conversion of angiotensin I to angiotensin II and also inhibits bradykinin metabolism thereby causing dilation of arteries and veins. These actions bring about reduction in preload and afterload on heart. Side effects of ACE inhibitors include cough, dysgeusia, hyperkalaemia, renal failure, proteinuria, agranulocytosis etc. Seldomly ACE inhibitors precipitate skin rashes, pruritus & urticaria.
Case report: A 52-year-old female with known case of rheumatoid arthritis was prescribed ramipril for treatment of cardiomegaly and left ventricular (LV) dysfunction. The patient reported skin related adverse drug reaction i.e., pemphigoid vulgaris.
Conclusion: From the current case report it can be said that ramipril can cause and aggregate some rare skin adverse effects as that of pemphigoid vulgaris which can be reversed within a short span of time if recognized early.
Keywords: ramipril; angiotensin-converting enzyme; pemphigoid vulgaris
Full Text
Introduction
Angiotensin-converting enzyme (ACE) inhibitors are very frequently used drugs in the treatment of hypertension, heart failure, diabetic nephropathy to prevent its progression. ACE inhibitors competitively inhibit the conversion of angiotensin I to angiotensin II and also inhibits bradykinin metabolism thereby causing dilation of arteries and veins.
ACE inhibitors acts in the following aspects: 1. Brings about dilation of arteries and veins by blocking angiotensin II formation & inhibition of bradykinin metabolism. This vasodilation reduces arterial pressure, preload and afterload on the heart. 2. Down regulate sympathetic adrenergic activity by blocking the facilitating effects of angiotensin II on sympathetic nerve release and reuptake of norepinephrine. 3. Promotes renal excretion of sodium and water (natriuretic and diuretic effects) by blocking the effects of angiotensin II in the kidney and by blocking angiotensin II stimulation of aldosterone secretion. As a result a reduction in volume of blood, venous pressure and arterial pressure is seen. 4. Cardiac and vascular remodeling associated with chronic hypertension, heart failure, and myocardial infraction is inhibited [1, 2].
Side effects of ACE inhibitors include cough, dysgeusia, hyperkalaemia, renal failure, proteinuria, agranulocytosis etc. Seldomly ACE inhibitors precipitate skin rashes, pruritus & urticaria. Other dermatological conditions include clinical events like that of onycholysis, hyperhidrosis, pemphigus, bullous pemphigoid, lichen planus, erythema multiforme & Stevens -Johnsons syndrome [3, 4].
This case report mainly focuses on a skin related adverse drug reaction (ADR) of ACE inhibitors and also impresses clinicians to be alert on such rare dermatological conditions/ manifestations while prescribing ACE inhibitor such as ramipril.
Case report
A 52-years-old female with known case of rheumatoid arthritis on Rx was referred to the cardiology department on 13 November 2018 for evaluation of cardiomegaly and left ventricular (LV) dysfunction associated with ischemia. The patient was assessed to have New York Heart Association (NYHA) class II and was prescribed following medications – aspirin 75 mg OD, atorvastatin 40 mg OD, ramipril 1.25 mg OD, spironolactone 25 mg OD, torsemide 10 mg BD & metoprolol 50 mg OD. On 1 June 2018 the dose of ramipril was increased to 2.5mg BD in the view of treating LV dysfunction. The patient complaint of blisters associated with itching (Figure 1) for which she was referred to the dermatologist. Dermatologist advised skin biopsy which revealed antidesmoglein I positive and thus was diagnosed as pemphigoid vulgaris. In view of drug induced pemphigoid vulgaris the patient was further referred to cardiology in opinion of altering ramipril. Ramipril was altered with losartan 12.5mg BD. After a month it was observed that the frequency and intensity of the appearing blisters was reduced (Figures 2 & 3).

Figure 1: (a, b) Sklin eruptions caused by ramipril.
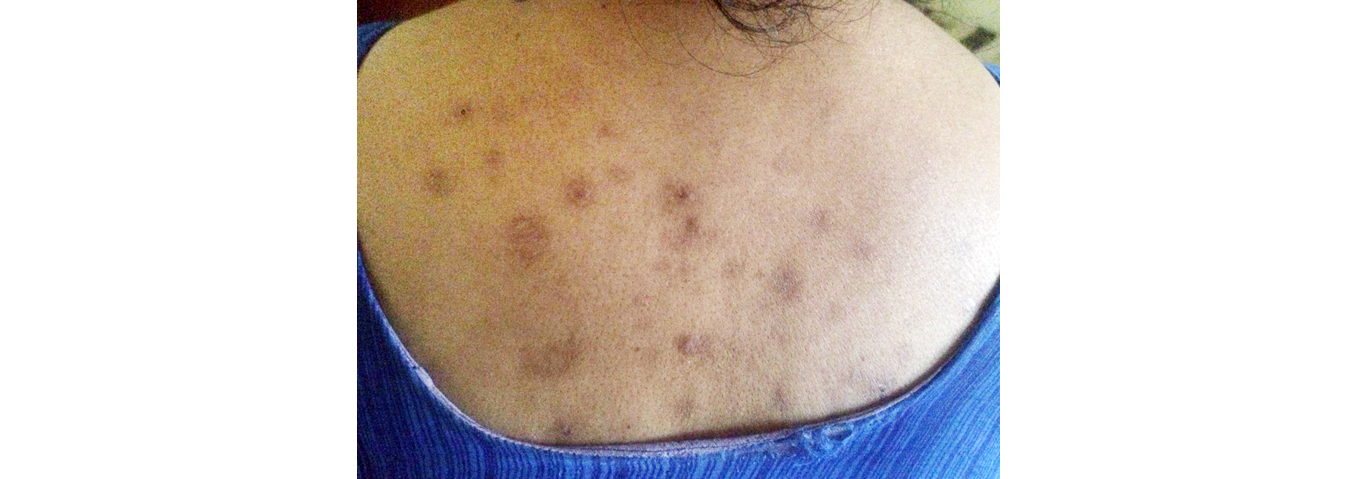
Figure 2: Subsided erruptions after alternating ramipril with that of losartan (after 2 months).
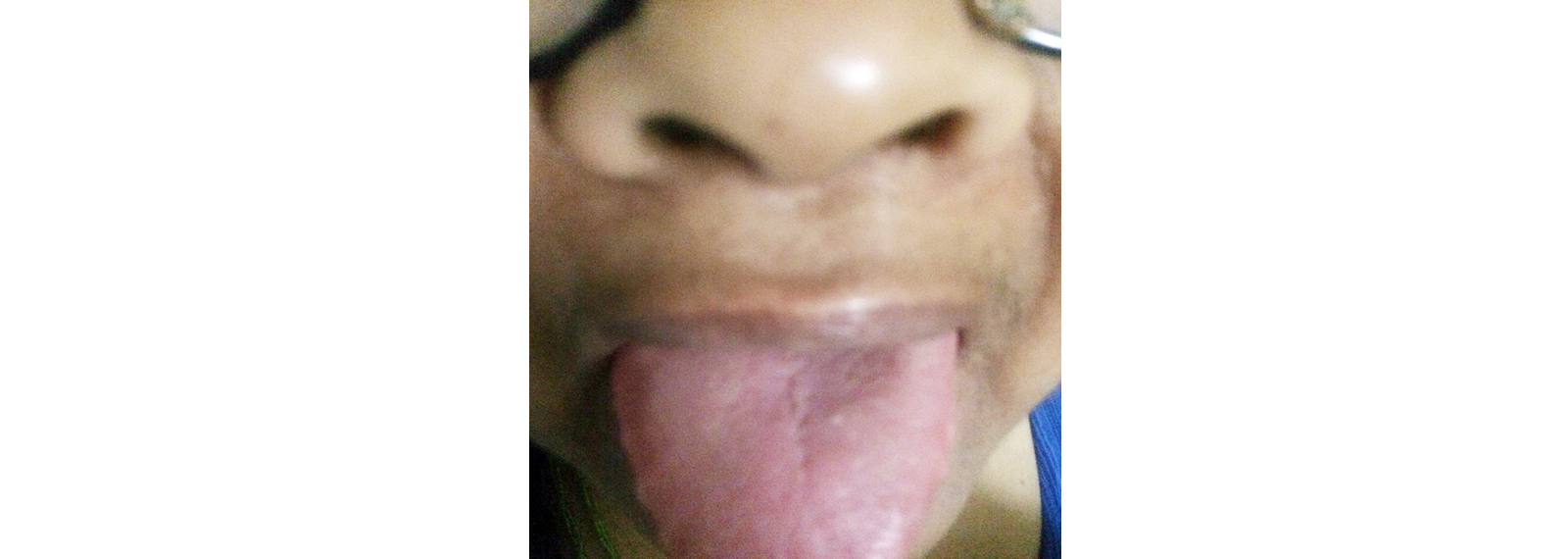
Figure 3: Eradication of oral pemphigus after altering ramipril with that of losartan.
Discussion
Pemphigoid vulgaris is an autoimmune disease which involves skin and mucous membranes causing painful blisters. It is the most common type of autoimmune disorder. It is mediated by circulating IgG antibodies directed against keratinocyte cell surface molecules desmoglein 3 (Dsg 3) and desmoglein 1 (Dsg 1) [5]. Interaction between the antibody and the desmoglein may have a direct effect on desmosal adherens and may also trigger a cellular process that results in acantholysis. The disease can develop spontaneously in majority of patients (idiopathic) or due to certain medication (drug induced pemphigus) [6].
Drugs that induce pemphigus can be categorised into two groups a) Thiol drugs & b) Non-thiol drugs. Thoil drugs are postulated to induce acantholysis through biochemical mechanisms without antibody formation. These usually include captopril, penicillamine & enalapril [7]. Non thiol drugs include sulfur containing drugs and drugs without sulfur in their structure. Sulfur containing drugs include penicillins, cephalosporins, antihypertensive agents and piroxicam. These drugs usually undergo hydrolytic breakdown in vivo to form thiols. Non thiols drugs are more likely to induce acantholysis via immune mechanisms [8].
Angiotensin converting enzyme is a zinc based metalloprotease. The enzyme is non-specific and its two major roles: 1. Regulation of local renin-angiotensin system; 2. Conversion of P-including bradykinin to inactive molecules [9, 10]. The mechanism for ACE inhibitor induced adverse reaction in the skin are mostly based on non-immunological mechanisms. Researchers have demonstrated the expression of a complete renin-angiotensin system in human skin including the precursor of angiotensin II, angiotensinogen, renin, angiotensin converting enzyme and receptors of AT1 & AT2 receptor subtype but their function is unknown [11]. Studies of cases of non-thiol induced pemphigus reveal the presence of autoantibodies that recognize pemphigus antigens, in particular desmoglein 3 [12]. Four cases of enalapril induced pemphigus have been reported [13]. Some cases of flares of pustular psoriasis with ramipril and captopril use in Psoriatic patients have been reported [14].
Conclusion
From the current case report it can be said that ramipril can cause and aggregate some rare skin adverse effects as that of pemphigoid vulgaris which can be reversed within a short span of time if recognized early.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Richard E Klabaunde. Cardiovascular pharmacology concepts. Available at: https://www.cvpharmacology.com.
[2] Sally Robertson Bsc. https://www.news-medical.net/health/ACE-Inhibitor-Mechanisms.
[3] Goodman and Gilmans. The pharmacological basis of therapeutics - 11th edition. 2006; pp:809.
[4] Mark L Glover. Martindle: The complete drug Reference. 35th edition. 2007; pp:1073.
[5] Brenner S, Goldberg I. Drug induced Pemphigus. Clin Dermatol. 2011; 29(4):455–457.
[6] Korman NJ, Eyre RW, Zone J, Stanley JR. Drug-induced pemphigus: Autoantibodies directed against phemphigus antigen complexes are present in penicillamine and captopril-induced Pemphigus. J Invest Dermatol. 1991; 96(2):273–276.
[7] Yoshimura K, Ishii N, Hamada T, Abe T, Ono F, et al. Clinical and immunological profiles in 17 Japanese patients with drug-induced pemphigus studied at Kurume University. Br J Dermatol. 2014; 171(3):544–553.
[8] Khashoggi M, Machet L, Perrinaud A, Brive D, Machet MC, et al. D-penicillamine-induced pemphigus: Changes in anti-32-2B immunostaining patterns. Ann Dermatol Venereol. 2013; 140(8-9):531–534.
[9] Steckelings UM, Wollshager T, Peters J, Henz BM, Hermes B, et al. Human Skin: Source of and target organ for Angiotensin II. Exp Dermatol. 2004; 13(3):148–157.
[10] Scholzen TE, Stander S, Riemann H, Brzoska T, Luger TA. Modulation of cutaneous inflammation by angiotensin converting enzyme. J Immunol. 2003; 170(7):3866–3873.
[11] Husgow T, Artuc M, Henz BM, et al. Normal skin as a potential source of angiotensin II. Arch Dermatol res. 1998; 290:49.
[12] Brenner S, Bialy-Golan A, Anhalt GJ. Recognition of pemphigus antigens in drug-induced pemphigus vulgaris and pemphigus foliaceus. J Am Acad Dermatol. 1997; 36(6 Pt 1):919–923.
[13] Kuechle MK, Hutton KP, Muller SA. Angiotensin converting enzyme inhibitors, pemphigus three case reports and literature review. Mayo Clin Proc. 1994; 69(12):1166–1171.
[14] Hamlet NW, Keefe M, Kerr RE. Does captopril exaberate Psoriasis? Br Med J (Clin Res Ed). 1987; 295 (6609):1352.