Full Text
Introduction
Stent thrombosis (ST) persists as one of the major limitations of coronary stent implantation. The incidence of stent thrombosis has been reduced by newer technologies and drugs, but mortality and morbidity when it occurs remains high.
Definitions: The definitions used for stent thrombosis were standardized by the Academic Research Consortium (ARC) [1, 2].
Stent thrombosis is defined as an abrupt onset of cardiac symptoms (i.e., an acute coronary syndrome) along with an elevation in levels of biomarkers or electrocardiographic evidence of myocardial injury after stent deployment [1, 2].
A definite stent thrombosis is accompanied by angiographic or autopsy evidence of a flow-limiting thrombus near a previously placed stent [1, 2]. When an acute coronary syndrome exists, although an angiogram cannot be performed, the event is called a probable or clinical stent thrombosis if another cause for the acute coronary syndrome is unlikely. Sudden death occurring within 30 days of stenting without angiography or autopsy is also probable stent thrombosis [1, 2].
A possible stent thrombosis refers to sudden death occurring after 30 days of stent implantation without angiography or autopsy [1, 2]. Acute stent thrombosis occurs within 24 hours of implantation. Early subacute thrombosis (SAT) occurs after one day up to 30 days of implantation. Late SAT occurs from 30 days to one year of implantation. Very late SAT occurs after one year of implantation. Acute and early subacute thrombosis together is classified as early stent thrombosis while the rest are categorized as late stent thrombosis [1, 2].
Incidence
The best available data comes from multiple registries [2]: 1) Bern-Rotterdam registry- 8146 patients underwent percutaneous coronary intervention (PCI). The cumulative incidence of angiographic stent thrombosis was 2.9%, with early (acute and subacute) stent thrombosis occurring in 60% and late stent thrombosis in 40%. After one year, the rate of stent thrombosis was 0.6% per year. 2) German-Italian registry-2229 patients who received either sirolimus eluting stents (SES) or paclitaxel eluting stents (PES). The rate of stent thrombosis was 1.3% (0.8% with SES and 1.7% with PES), with 0.6% occurring in 30 days and 0.7% beyond 30 days. 3) In a prospective observational study that used the ARC definition, definite or probable ST occurred in 1.9% patients who received drug eluting stents (DES). Of these patients, 72% sustained an event within six months of the procedure. 4) Swedish Coronary Angiography and Angioplasty Registry (SCAAR)-42,150 patients underwent PCI with either bare metal stents (BMS) or DES. The rate of definite stent thrombosis was 1.2%, of which 50% were acute or subacute. The rate after one was 0.3 to 0.4% per year. 5) Dutch stent thrombosis registry- 21,009 underwent PCI. ARC definite stent thrombosis was present in 2.1% of which 32% was acute, 41% subacute, 13% late and 14% very late. 6) ESTROFA registry- 23500 DES implanted patients. 301 (1.3%) stent thrombosis were reported, 149 early, 90 late and 62 very late thrombosis. 6) Denmark registry- 12395 patients. Overall stent thrombosis incidence was 1.8 per 1000 cases per year for BMS compared to 1.5 per thousand for DES. 7) Kastrati and Schomig- Meta-analysis of 17 randomised trials. The total ST in BMS implantations was 37 out of 2811 and in DES implantations were 38 out of 2795.
The incidence of ST depends on the type of stent used. While older DES (first generation) had a comparatively high incidence of ST, newer stents (2nd and 3rd generation) have a lower incidence. A great deal of data has been accumulated on ST occurrence in different types of DES.
Bare metal vs drug eluting stents
The differences in the characteristics of ST between BMS and DES has generated a great deal of interest. The incidence of early thrombosis is similar for BMS and DES. Late thrombosis occurs with significantly higher incidence in DES. Late thrombosis after BMS has a very low incidence with the exception of intra-coronary irradiation used to reduce the incidence of in-stent re-stenosis [2, 3].
Drug eluting stents
A. First generation stents: 1) Cypher SES- The j-Cypher registry followed patients for 5 years after SES implantation. The incidence of ST was 0.3% at 30 days, 0.6% at 1 year and 1.6 % at 5 years. Importantly, there was a continuing incidence of very late ST up to 5 years after implantation at a rate of 0.26%/yr. This data has led to the recommendation that dual anti-platelet therapy be continued indefinitely for patients implanted with Cypher SES [4]. 2) TAXUS PES- A meta-analysis of the TAXUS II, IV, V & VI studies showed that 0.8% of patients have ST after TAXUS stent implantation in the first 6 months. This incidence is similar to BMS. From 6 months to 3 years however, the incidence of ST in PES rose to 1.28% ± 0.31% in the PES group compared to 0.76% ± 0.23% in the BMS group. This observation is believed to be related to clopidogrel discontinuation after 6 months [6].
B. Second generation stents: 1) Xience V everolimus eluting stents (EES)- The Xience V United States Study followed patients for a period of one year. The incidence of ST was 0.8% [7]. A European study of over 10,000 patients was presented at EuroPCR 2012, which showed that the incidence of ST with Xience V and Xience Prime EES is unchanged after discontinuation of dual anti-platelet therapy after 3 months. This is the shortest duration of dual antiplatelets recommended in Europe for any DES at the current time [8]. 2) Resolute and Endeavorzotaroliumus eluting stents (ZES)- Two trials (ISAR TEST-2 and ISAR TEST-5) followed patients implanted with these ZES platforms. The incidence of ST at 2 years was 0.4% for Resolute ZES and 0.6% for Endeavor ZES [9]. 3) Promus element SES- Jose et al concluded that the incidence of ST in patients implanted with platinum-chromium SES was 0.66% at the end of 18 months. Their data was similar to the PLATINUM study which showed non-inferiority of this stent compared to cobalt-chromium EES [10].
C. Third generation stents: 1) Biomatrixbiolimus eluting stents (BES)-Biomatirx uses a biodegradable polymer. The results of the e-Biomatrix registry were presented in EuroPCR 2013. 5559 patients were studied. The incidence of ST at the end of one year was 0.6% with most incidences occurring in the first month [11]. 2) Bioabsorbable Scaffolds- Bioabsorbable scaffolds are likely to prove to be the future of coronary angioplasty. So far, the results have been promising. Only a small number of cases of ST have been reported till date. Since the scaffold gets totally absorbed, it is possible that very late ST will be completely eliminated [12].
Predictors of stent thrombosis
Predictors of stent thrombosis are described in Table 1 [1-3, 13]. The predictors can be classified into three categories: patient characteristics, lesion characteristics and procedural characteristics.
Table 1: Predictors of stent thrombosis
|
Patient characteristics
|
Lesion characteristics
|
Procedural characteristics
|
|
Diabetes mellitus
|
Long segment disease
|
Stent under expansion
|
|
Chronic kidney disease
|
Small diameter vessel
|
Stent malapposition
|
|
Acute coronary syndrome
|
Saphenous vein graft
|
Edge dissection
|
|
Current smoker
|
Chronic total occlusion
|
Strut fracture
|
|
Cancer
|
Bifurcation lesions
|
Multiple stent implantation and overlap
|
|
Resistance to anti-platelet therapy
|
|
Lesion miss
|
|
Premature cessation of anti-platelets
|
|
Residual stenosis
|
|
Advanced age
|
|
Slow flow/ no flow after procedure
|
|
Thrombocythemia
|
|
Intra-coronary irradiation
|
|
Hypersensitivity to polymer or drug
|
|
|
Patient characteristics which predispose to ST include conditions which are related to increase in atherosclerotic activity and plaque instability. The most common conditions seen are diabetes mellitus, acute coronary syndrome, kidney disease, smoking, advanced age and cancer. Rarer conditions are thrombocythemia, resistance to anti-platelet agents and hypersensitivity to the stent drug or polymer.
Certain lesions are more predisposed to ST. These include long lesions, lesions in small diameter vessels, chronic total occlusion, saphenous vein graft lesions and bifurcation lesions. This increased propensity to ST is independent of the technical difficulty of performing an optimal procedure on these lesions.
Procedural characteristics are potentially avoidable. The most important factors are stent under-expansion, stent mal-apposition and edge dissection. Other factors are strut fracture, usage of multiple overlapping stents, missing part of the lesion and leaving residual stenosis. Slow flow and no reflow phenomenon is a risk factor for ST. Several strategies are currently under evaluation to prevent and treat this problem. Intra-coronary irradiation reduces the risk of in-stent restenosis, but results in a high incidence of ST. This factor is now rare as intra-coronary irradiation usage has reduced across the world.
Mechanisms
Early stent thrombosis: The mechanisms or early thrombosis are mainly procedure related. The commonest cause is residual dissection. Other frequent causes are stent under expansion or malappostion, persistent thrombus, tissue prolapsed, slow flow and left ventricular dysfunction [2, 3].
Late stent thrombosis: The primary mechanism for late thrombosis is incomplete epithelialisation of the intima over the stented segment. With BMS, intra-coronary irradiation used to be the major cause of this phenomenon. For DES, the non-absorbable polymer hampers epithelialisation. Since epithelialisation can be hampered for a year or even more, prolonged dual anti-platelet therapy is required. Other causes for hampered epithelialisation include long stents, long overlap zone, bulky stent struts, bifurcation stenting (due to multiple layers of struts) and stent crossing side branch (struts cross the branch origin and remain bare). Interestingly, diffuse in-stent restenosis has been shown to be a risk factor for SAT. Another interesting finding is the high incidence of stent thrombosis reported after stenting over an area containing a lipid rich unstable plaque. This finding partially explains the higher incidence of thrombosis in ACS and underlines the recommendation that a non-significant lesion not be stented even if it is an unstable plaque [2, 3, 13].
Trial data on BMS vs DES: The major trial data is shown in Table 2 [2]. Though the incidence of SAT is higher for DES compared to BMS, the overall benefit of DES over BMS persists. Sirolimus eluting stents (SES) seem to have a lower incidence of SAT compared to paclitaxel eluting stents (PES). Along with the higher incidence of SAT, the time course of SAT is markedly prolonged for DES compared to BMS and higher for PES compared to SES (Figure 1) [2, 3].
Table 2: Trials comparing BMS Vs DES.
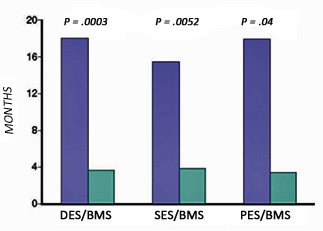
Figure 1: The course for drug-eluting stent (DES) thrombosis is markedly protracted compared with that for a bare metal stent (BMS). PES, pacitaxel-eluting stent; SES, sirolimus-eluting stent. (From Bavry AA, Kumbhani DJ, Helton TJ, et al; Late thrombosis of drug eluting stents: A meta-analysis of randomized clinical trials. Am J Med 2006;119:1056-1061.)
Effect of new stent technology
New stent technologies are under constant development. The aims of developing new stents include intra-procedure technical ease, reduction in incidence of stent thrombosis, reduction in incidence of in-stent restenosis and reduction in duration of mandatory dual anti-platelet therapy.
Second and third generation stent [14]
Second generation stents have been in use for several decades now. The major difference between 1st and 2nd generation stents is the strut design and polymer. While 1st generation stents use 316 L stainless steel scaffold, 2ndand 3rd generation stents use cobalt chromium or platinum chromium struts which have similar radial strength with thinner struts. Thinner struts reduce stent thrombosis, facilitate endothelialisation and decrease thrombogenicity.
Newer polymers have been used in these stents like phosphoryl choline in ZES and fluoropolymer in EES. These polymers result in less inhibition of endothelialisation and reduced inflammation. However, the congeners of sirolimus like zotarolimus and everolimus used in several of these stents have not shown any major advantage over sirolimus Dores et al have reported from an analysis of a large real-world registry on the incidence of ST in first generation versus second generation stents. They state that the incidence of ST is almost 2.4 times higher in first generation stents (Figure 2). The ESTROFA-2 registry showed similar data and also found a higher rate of ST with zotarolimus eluting stents used for bifurcation lesions compared to everolimus eluting stents [15].
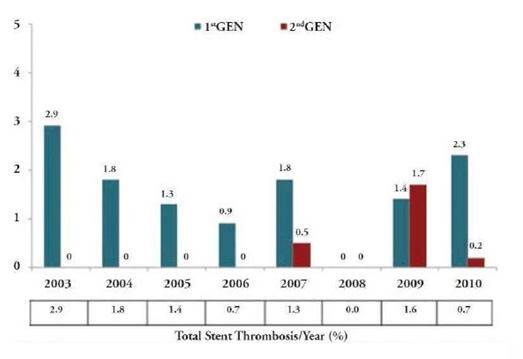
Figure 2: Rate of definite stent thrombosis according to year of the index procedure and study groups.
Importantly, from the total of ST cases, 69.6% (n=32)occurred in patients treated in the setting of an ACS (P<.001) and the majority (n=24; 52.2%) were late ST (occurring between 30 days and 12 months). For all time periods, according to the ARC consensus, the incidence of the primary endpoint was always numerically superior in the 1st GEN group, but a statistically significant difference was detected only in the case of late ST: acute ST was 0.3% Vs 0.1% (P= .104), subacute ST was 0.4% Vs 0.2% (P= .289), and late ST rate was 0.8% Vs 0.3% (P= .036).
Different strategies with stent polymers [14]
Various strategies have been tried with stent polymers to reduce the incidence of ST.
Stents with bio-degradable polymer have been shown to significantly reduce the incidence of ST6.Since the polymer degrades over a period of a year, the patient gets the benefit of a DES with the reduced incidence of ST as seen in a BMS after one year. Biolimus eluting stents with bio-degradable polymer and SES with bio-degradable polymer are available and are increasingly used. The LEADERS, ISAR-TEST 3 and ISAR-TEST 4 studies have shown favourable results with use of bio-degradable polymers. A meta-analysis of these three studies showed and definite reduction in incidence of ST at 3 years.
Polymer free DES may be associated with reduced ST. However, a major concern with these stents is the rapid release of drug, with possible high late loss over the short term. Dual polymer free DES (sirolimus with probucol) may overcome this limitation.
Novel polymer free DES use several approaches to store the drug. Several approaches have been examined, including microtextured stainless steel reservoirs in cobalt chromium struts and carbon coated slotted struts. The Yukon stent (Translumina, Hechingen, Germany) has a roughened stent surface to which a drug solution can be applied in the catheterization laboratory. The BioFreedom stent (Biosensors International, Singapore) is a stainless steel scaffold modified by microabrasion to create a highly textured abluminal surface. This allows drug (Biolimus A9) adhesion to the stent’s abluminal surface without the use of a polymer.
Bioabsorbable scaffold [14, 16]
Complete bioabsorbable scaffolds have recently been approved for use. Early data show promise in significantly reducing rates of ST and ISR. Potential advantages over currentDES are as follows: 1) the removal of the scaffold facilitatesthe return of vessel vasomotion, adaptive shearstress, late luminal enlargement, and the reduction of scaffoldthrombosis; 2) a reduction in the requirements forlong-term dual anti-platelet therapy, thereby reducing thebleeding complications; 3) allowance for future revascularizationand the use of noninvasive imaging techniquessuch as computed tomography or magnetic resonance imaging for follow-up.This may well prove to be the technology of the future.
Novel stent coatings [14]
The Genous stent (OrbusNeich, Fort Lauderdale, FL, USA) has an abluminal coating of the CD34 antibody on a bare metal stainless steel scaffold which sequesters circulating endothelial cell progenitors from the blood stream in an attempt to accelerate endothelialization and reduce stent thrombosis. This stent showed a higher rate of vessel occlusion compared with DES in the 1st year. However, this approach has been combined was used in a novel stent (Combo Dual Therapy stent; OrbusNeich, Fort Lauderdale, FL, USA) combining CD34 antibody technology with the antiproliferative agent, sirolimus, and a biodegradable polymer coating. It has recently been approved for use in Europe.
The Titan-2 BioActivestentTM (Hexacath, Paris, France) is constructed from stainless steel coated in titanium nitric oxide. Nitric oxide is an endogenous signaling molecule that induces vasodilatation and inhibits both platelet aggregation and smooth muscle cell proliferation. A number of initial small studies have demonstrated that the Titan-2 stent was superior to conventional BMS,and equivalent to paclitaxel (TITAX-AMI) and everolimus eluting stents, at reducing in-stent late loss.
In addition to nitric oxide donors, stents coated with genetic information targeting nitric oxide metabolism are in evaluation. As an example, stent struts coated with lipopolyplexes expressing nonviral plasmid DNA encoding endothelial nitric oxide synthase, an enzyme that catalyzes the production of nitric oxide from L-arginine, have been shown to inhibit neointimal hyperplasia.An in vivo study using stents coated with lipopolyplexes containing endothelial nitric oxide synthase DNA demonstrated accelerated endothelialisation. Whether this strategy will be successful in patients remains to be determined.
Micromesh covered stent [17]
The micromesh covered MGuard stent (InspireMD, Tel Aviv, Israel) has been designed for use in acute STEMI and reduces the incidence of slow flow and no-flow phenomenon. It is hoped that reduction of the incidence of this risk factor for ST will reduce the risk of ST at least in the early phase.
Dedicated Bifurcation stents [17]
Dedicated bifurcation stents designed either for stenting of the side branch without missing the ostium or for maintaining access to the side branch after main vessel stenting have been approved. It is conceivable that the better expansion and lesion covering provided by these stents without multiple layers of metal within the vessel may reduce the incidence of ST.
Choice of anti-platelet agents
The choice of dual anti-platelet therapy has an effect on ST incidence as well. Aspirin is given to all cases across the board. Till recently, clopidogrel was the standard and mandatory anti-platelet agent to be used in all cases for duration depending on the stent used. The newer anti-platelet agents have shown superiority over clopidogrel in preventing all the varieties of ST.
Prasugrel is a new thienopyridine agent which is now recommended for use after stenting as a class I indication by the ACC/AHA and the ESC guidelines. The TRITON TIMI-38 trial showed a 59% reduction in the incidence of all varieties of ST with use of prasugrel irrespective of stent type compared to clopidogrel [18].
Ticagrelor is a novel anti-platelet agent which has been recommended for use across the board in patients with acute coronary syndrome. The PLATO trial showed a significant reduction in incidence of ST across the board with ticagrelor compared to clopidogrel [19].
Outcome of stent thrombosis
Stent thrombosis frequently has a catastrophic outcome. The e-Cypher registry reported a 42% mortality incidence and a 44% incidence of myocardial infarction due to SAT after SES implantation. Iakovou et al reported a 45% mortality following stent thrombosis [2].
Management of stent thrombosis
There is still no clear consensus on the best method of treating ST. Aggressive supportive management is mandatory. Fibrinolysis has been used in some centres with success especially in case of early presentation [20].
Re-intervention is currently preferred if available. The best practice is still uncertain. Various methods tried include thrombus aspiration, balloon angioplasty, re-stenting with second or third generation stent and even CABG. Ancillary drugs used include GP IIb/IIIa antagonists and bivaluridin [2].
Prevention of stent thrombosis
No method can completely prevent ST. However, it is possible to minimise the incidence of ST with certain precautions [2].
A. Stent selection and deployment: 1) Second and third generation stents reduce the incidence of ST. Polymer free and bio-absorbable polymer stents reduce ST as well. 2) Stent deployment must be optimised. If necessary, IVUS or OCT can be used to confirm adequate stent expansion. 3) Overlap zones should not be longer than necessary. 4) Dissection and strut fracture should be looked for after the procedure. 5) Usage of GP IIb/IIIa antagonists reduces the incidence of ST. 6) Bivaluridin has been reported to reduce ST incidence.
B. Anti-platelet therapy: 1) Dual anti-platelet therapy should be continued for at least 12 months if not contraindicated. As mentioned above, Xience V and Xience Prime EES have been approves for use with 3 month dual anti-platelet therapy in Europe [8]. 2) Prasugrel or ticagrelor are preferable if there is no contra-indication. 3) It is safer to avoid elective surgeries for 12 months after DES implantation. Emergency surgeries should be done under aspirin cover.
Future directions
Several new technologies and drugs are in development and evaluation and show promise in reducing ST incidence. While the bioabsorbable scaffold is already in the market, stents with novel coating agents are under evaluation. New drugs, like otomixoban and cangrelor, are under evaluation.
Points to remember
1) Optimum stent sizing and deployment should always be aimed for during angioplasty. 2) Use of prasugrel and ticagrelor reduce the incidence of ST. 3) It may be preferable to use BMS in patients who are invariably going to undergo any surgery in the 1st year after stent implantation. The problem of higher rates of restenosis should be considered before decision making. 4) Complex angioplasty is a predictor for ST and these patients should be monitored. 5) Use of GP IIb/IIIa inhibitors or bivaluridin may reduce the incidence of ST especially in the setting of ACS.
Conclusion
Stent thrombosis is still one of the major challenges in coronary intervention. Several predictors of high risk for ST have been defined, but ST can occur in the absence of any identifiable risk factor. ST can be managed by aggressive medical and interventional therapy. Proper care during angioplasty, new drugs and next generation stents are reducing the incidence of ST, but the problem is not yet eliminated.
Conflicts of interest
Authors declare no conflicts of interest.
References
1. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007; 115(17):2344-2351.
2. Gupta S, Gupta MM. Stent Thrombosis. J Assoc Physicians India. 2008; 56:969-979.
3. Bavry AA. Late Stent Thrombosis. Topol-Textbook of Interventional Cardiology 5ed, 2008, Philadelphia, Chap 31, 549-566.
4. Kimura T, Morimoto T, Nakagawa Y, Kawai K, Miyazaki S, et al. Very late stent thrombosis and late target lesion revascularization after sirolimus-eluting stent implantation: five-year outcome of the j-Cypher Registry. Circulation. 2012; 125(4):584-591.
5. Ellis SG, Colombo A, Grube E, Popma J, Koglin J, et al. Incidence, timing, and correlates of stent thrombosis with the polymeric paclitaxel drug-eluting stent: a TAXUS II, IV, V, and VI meta-analysis of 3,445 patients followed for up to 3 years. J Am Coll Cardiol. 2007; 49(10):1043-1051.
6. Naidu SS, Krucoff MW, Rutledge DR, Mao VW, Zhao W, et al. Contemporary incidence and predictors of stent thrombosis and other major adverse cardiac events in the year after XIENCE V implantation: results from the 8,061-patient XIENCE V United States study. JACC Cardiovasc Interv. 2012; 5(6):626-635.
7. Abott Vascular. Data presented in Euro PCR 2012.
8. Tada T, Byrne RA, Cassese S, King L, Schulz S, et al. Comparative efficacy of 2 zotarolimus-eluting stent generations: resolute versus endeavor stents in patients with coronary artery disease. Am Heart J. 2013; 165(1):80-86.
9. De la Torre Hernandez JM, Garcia Camarero T, Lerena P, Lee DH, Sainz Laso F, et al. A real all-comers randomized trial comparing Xience Prime and Promus Element stents. J Invasive Cardiol. 2013; 25(4):182-5.
10. E-Biomatrix Registry. Data presentation at Euro PCR 2013.
11. Ormiston JA, Serruys PWS. Bioabsorbable coronary stents. Circulation. 2009; 2(3):255-260.
12. Cheneau E, Leborgne L, Mintz GS, Kotani J, Pichard AD, et al. Predictors of subacute stent thrombosis: results of a systematic intravascular ultrasound study. Circulation. 2003; 108(1):43-47.
13. Kim HK, Jeong MH. Coronary stent thrombosis: current insights into new drug-eluting stent designs. Chonnam Med J. 2012; 48(3):141-149.
14. Dores H, Raposo L, Campante Teles R, Machado C, Leal S, et al. Stent thrombosis with second- versus first-generation drug-eluting stents in real-world percutaneous coronary intervention: analysis of 3806 consecutive procedures from a large-volume single-center prospective registry. J Invasive Cardiol. 2013; 25(7):330-336.
15. Patel N, Banning AP. Bioabsorbable scaffolds for the treatment of obstructive coronary artery disease: the next revolution in coronary intervention? Heart. 2013; 99(17):1236-1243.
16. Wilson WM, Cruden NLM. Advances in coronary stent technology: current expectations and new developments. Res Rep Clin Cardiol. 2013; 4:85-96.
17. Capranzano P, Ferreiro JL, Angiolillo DJ. Prasugrel in acute coronary syndrome patients undergoing percutaneous coronary intervention. Expert Rev Cardiovasc Ther. 2009; 7(4):361-369.
18. Steg PG, Harrington RA, Emanuelsson H, Katus HA, Mahaffey KW, et al. Stent thrombosis with ticagrelor versus clopidogrel in patients with acute coronary syndromes: an analysis from the prospective, randomized PLATO trial. Circulation. 2013; 128(10):1055-1065.
19. Sciahbasi A, Patrizi R, Madonna M, Summaria F, Scioli R, et al. Successful thrombolysis in patients with subacute and late stent thrombosis. Can J Cardiol. 2009; 25(6): 213-214.
20. Medina RP, Foto D. The use of bivalirudin to prevent subacute thrombosis during drug-eluting stent implantation. J Invasive Cardiol. 2004; 16(5):236-239.