Full Text
Introduction
Giant cell tumour is a locally aggressive benign bone tumour which accounts for 30% of primary bone tumours in south India [1]. The incidence of recurrence after primary therapy with simple intra lesional curettage of giant cell tumour of bone (GCTB) varies from 32-69% (2–4). Use of adjuvants like bone grafting, H2O2, phenol, liquid nitrogen and PMMA has brought down the recurrence to 9-22% [2-5]. This incidence is further reduced to 12-14% with the use of high speed burr and bone grafting [6]. A more effective method of curettage is to remove 5 mm of normal bone all around the tumour, which is referred to as “extended curettage” by us and this reduced the incidence to 8% [7]. Removal of 5mm of normal bone is not possible towards the joint when the tumour extends up to the subchondral bone. This increases the chances of recurrence even after an effective curettage. Further, recurrence of the tumour depends not only on the efficacy of curettage but also on the aggressiveness of the tumour. Hence, recurrence is a challenging complication of GCTB treatment. Recurettage of a GCTB does not guarantee no further recurrence. Re-recurrence rate was reported as 21.7% by Balke et al. [2].
In this scenario we need a molecule which inhibits the lysis of bone so that osteoblastic activity dominates. Denosumab is such a recent innovation.
We report two cases of GCTB where denosumab was used; in one case after second recurrence and in the other after first recurrence and the results were encouraging.
Case 1
A 17-year-old female presented to our outpatient Department on 13/06/2015 with pain over left distal thigh for 3 months. She gave a history of GCTB of left distal femur. Curettage and bone cement filling was done in China one year earlier (Figure 1).
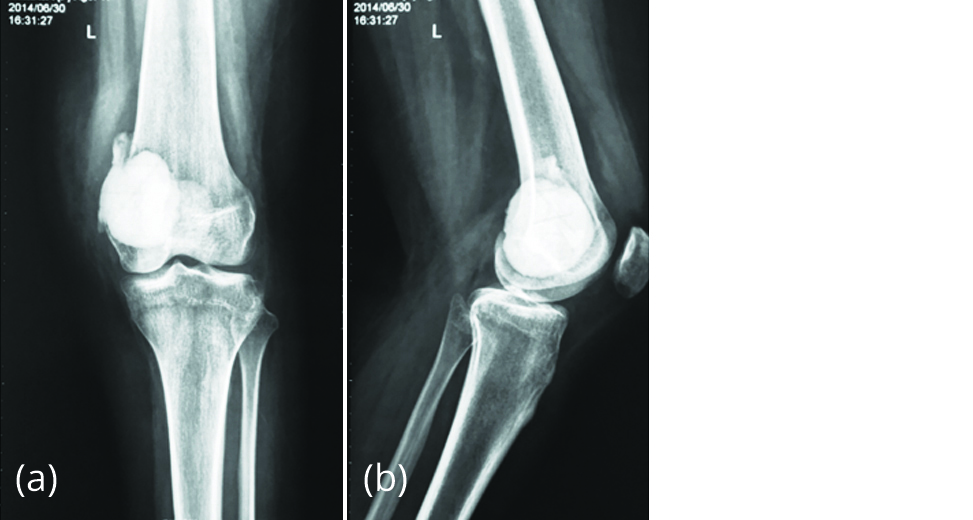
Figure 1a, b: Radiograph of left knee post-surgery showing bone cement in the distal femur.
Presenting radiograph (Figure 2) showed lytic reaction around bone cement with an intact articular surface, suggesting recurrence of tumour.
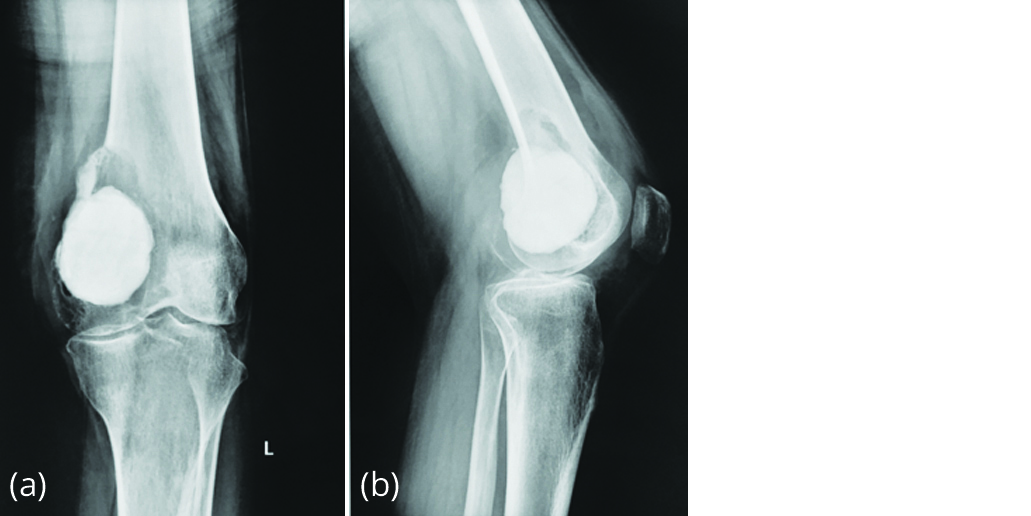
Figure 2a, b: Radiograph in June 2015 showing lytic lesion on top of bone cement.
On 15/06/2015, bone cement was removed and extended curettage of lesion was done. H2O2 and liquid nitrogen were used as adjuvants. The cavity was filled with autogenous fibula strut and allogenous cancellous bone chips (Figure 3). Histology of the curettings revealed giant cells and tumour stromal cells suggestive of recurrence of giant cell tumour of bone.
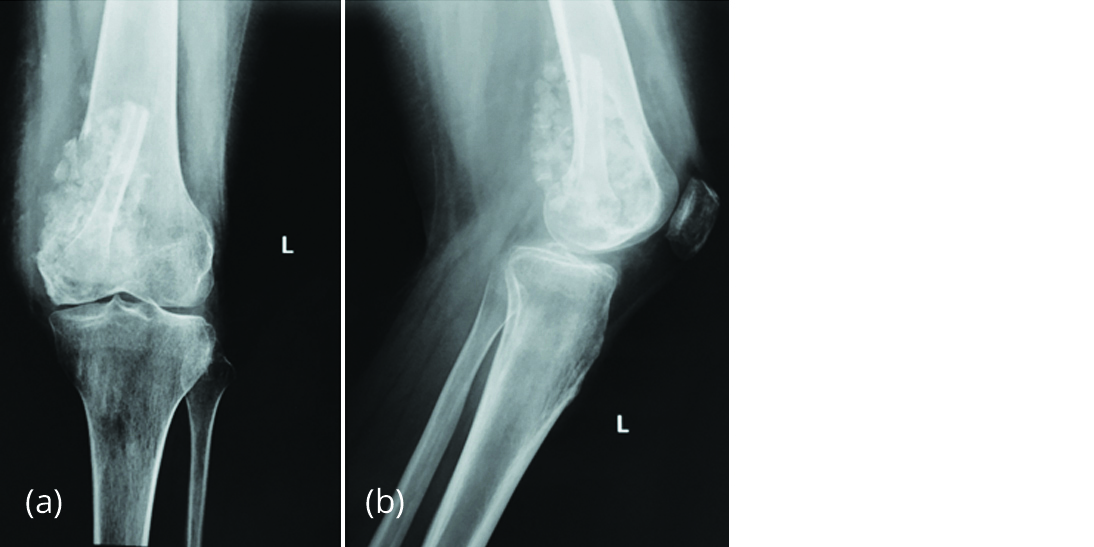
Figure 3a, b: Radiograph of left knee after curettage showing fibula strut and cancellous bone graft.
The patient presented with pain in left distal thigh 18 months later and the radiology suggested recurrence of GCT (Figure 4).
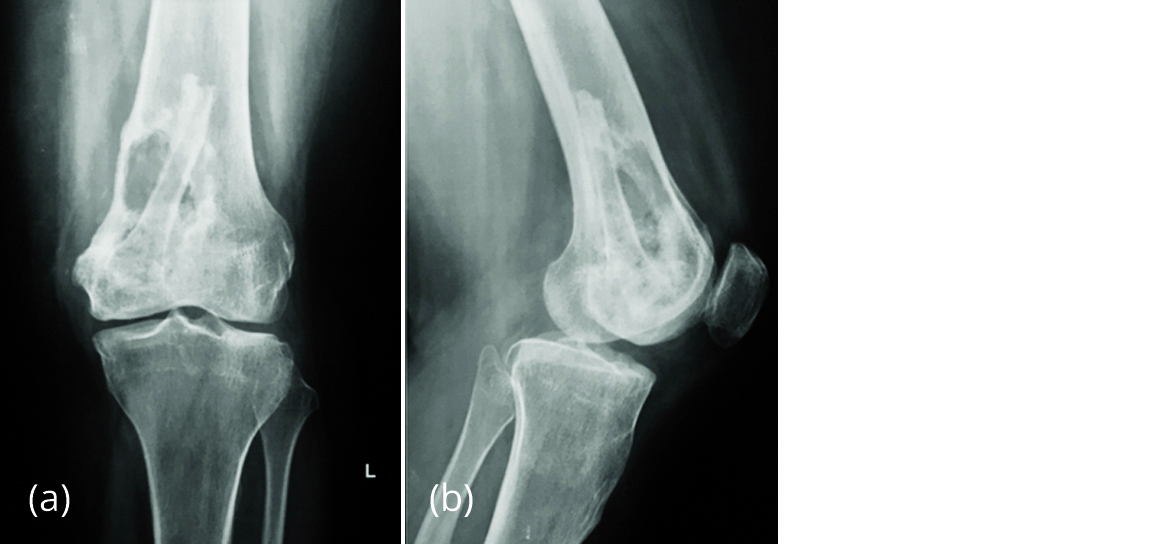
Figure 4a, b: Radiograph of left knee in Feb 2017 showing lytic lesion in distal femur with fibular graft in situ.
Recurettage was planned but patient was not willing for surgery at that time and presented to the OPD after 5 months with increased pain in distal thigh. Radiograph showed increase in the size of lesion with partial absorption of fibular graft (Figure 5). Ultrasound guided wide needle biopsy of the lesion was suggestive of recurrence of GCT (Figure 6).
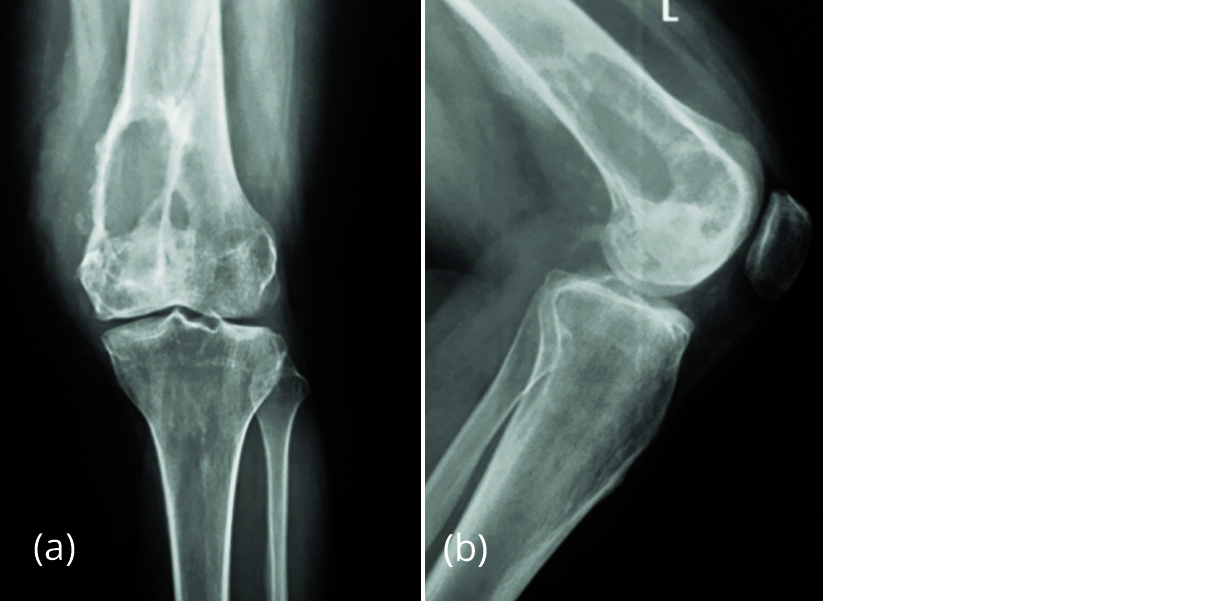
Figure 5a, b: Radiograph in July 2017 showing increase in size of lesion with partial absorption of fibular graft.
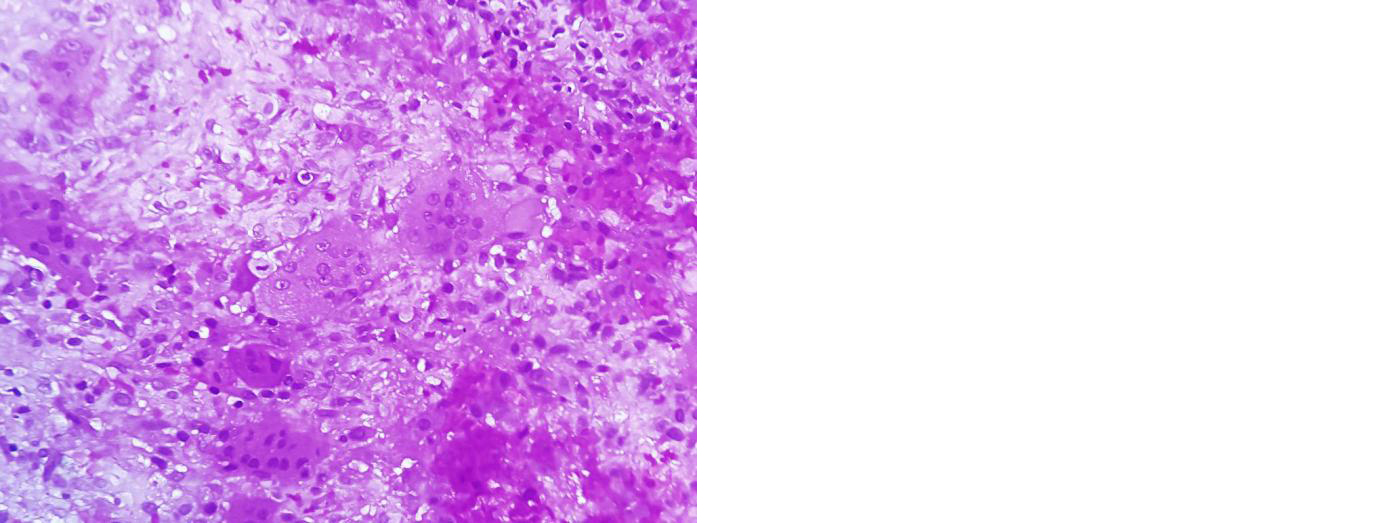
Figure 6: Section shows evenly spaced osteoclastic giant cells in a cellular stroma composed of round to oval stromal cells containing bland nuclei. Nuclei of stromal cells resemble nuclei of giant cells suggestive of giant cell tumour.
As the patient was not ready for the surgery, after reviewing literature we started her on subcutaneous inj denosumab 120mg with loading doses on day 1, 8, 15, 30 and later every 4 weeks for 6 months. Patient was followed up every month and at the end of 6 months, patient improved clinically with no pain. Radiograph left knee showed significant sclerosis and decrease in size of lesion (Figure 7).
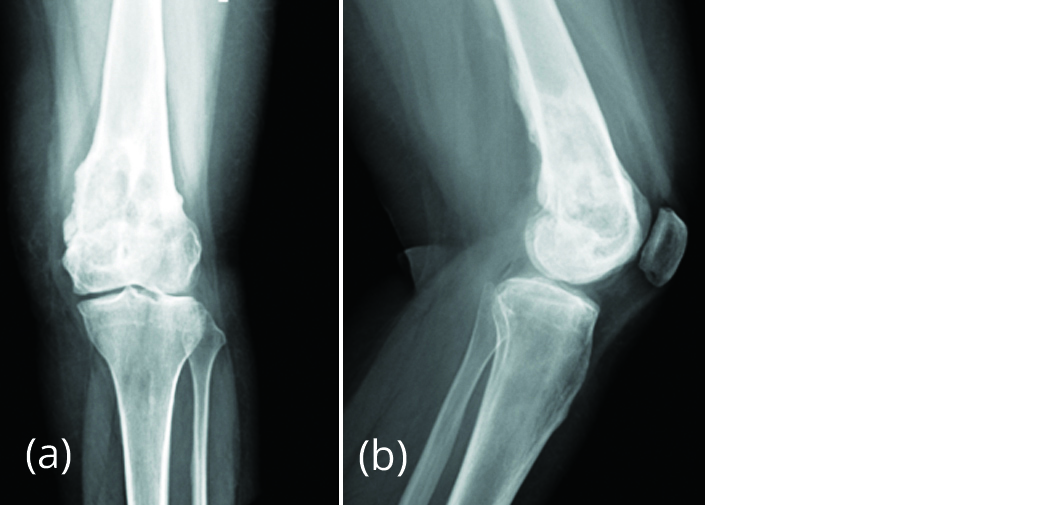
Figure 7a, b: Radiograph of left knee, post denosumab therapy for 6 months showing new bone formation and regression of size of lesion.
CT scan of the lesion showed a cavity, we were inquisitive to know the contents of the cavity and curetted it and void filled with cancellous bone graft. Contents of the cavity were found to be gritty in nature. Histology of the tissue revealed fibrous dysplasia like appearance with only woven bone trabeculae (Figure 8). No giant cells or stromal cells were found.
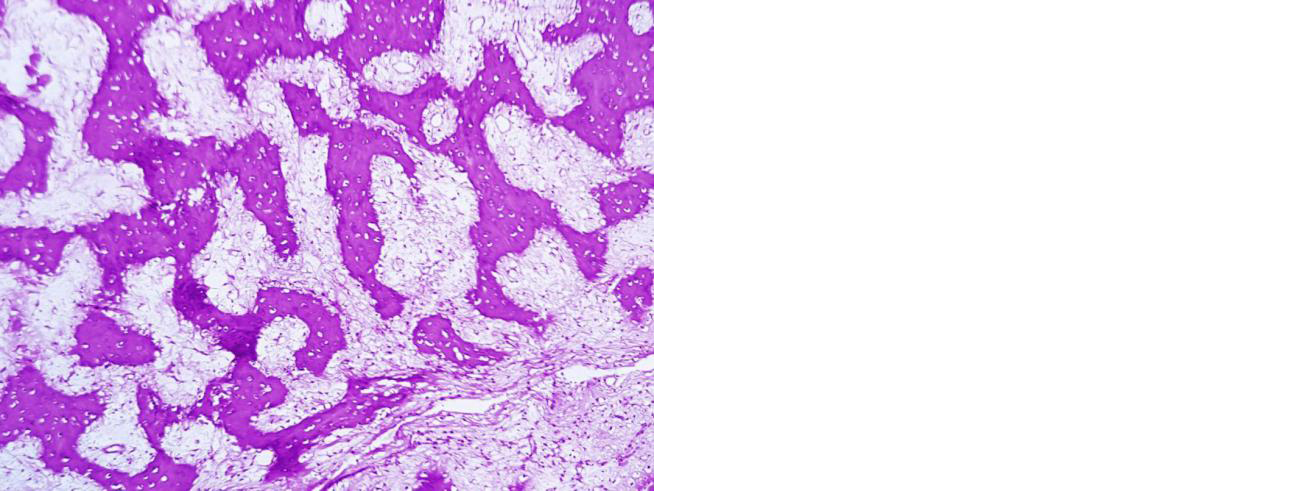
Figure 8: Section showing new bone formation and absence of giant cells and stromal cells. 12 months post denosumab therapy, she does not have any clinical and radiological signs of recurrence (Figure 9).
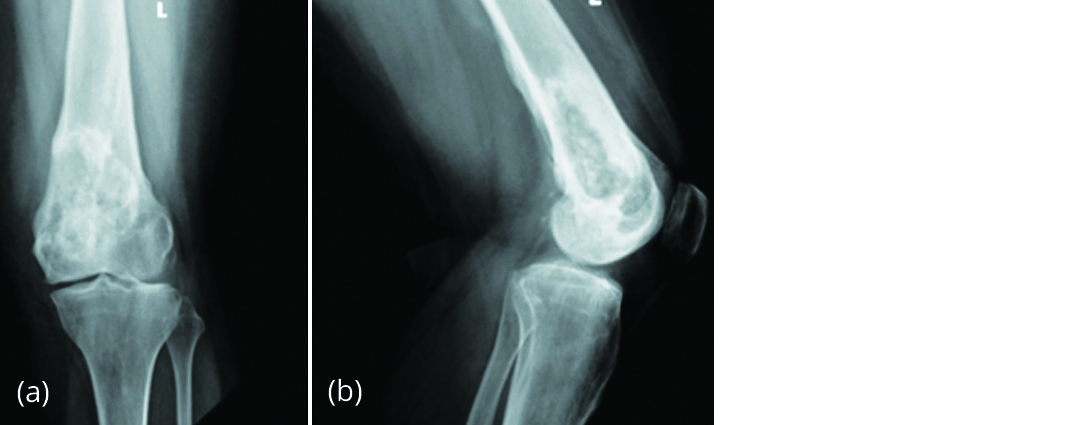
Figure 9a, b: Radiograph of left knee after 12 months post denosumab therapy shows increase in bone density and no signs of recurrence.
Case 2
A 20-year-old female presented to our outpatient Department with complaint of pain in left ankle of 2 months duration with no history of trauma or fever. On examination, anterior surface of left distal tibia was tender and ankle movements were restricted. Radiograph of left ankle (Figure 10) showed a benign lytic lesion in distal tibia extending up to the subchondral area with breach of the anterior cortex suggestive of giant cell tumour.
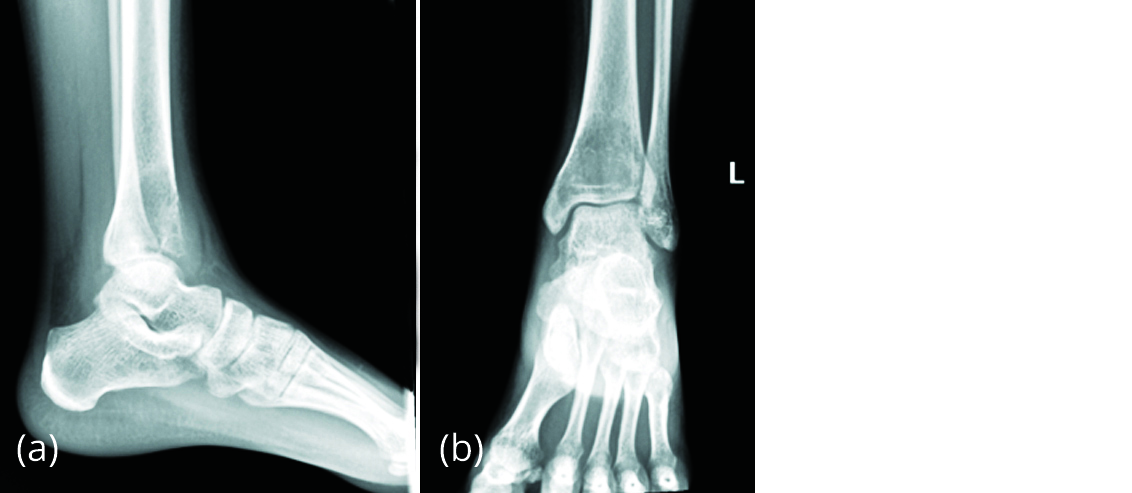
Figure 10a, b: Radiograph of left ankle showing eccentric, lytic lesion in distal tibia suggestive of GCT.
Needle biopsy revealed a giant cell containing lesion. She underwent an extended curettage with H2O2 and liquid nitrogen as adjuvants. The cavity was filled with allograft cancellous bone chips (Figure 11). Histology of contents showed mononuclear stromal cells and spatially arranged multinucleate giant cells consistent with giant cell tumour.
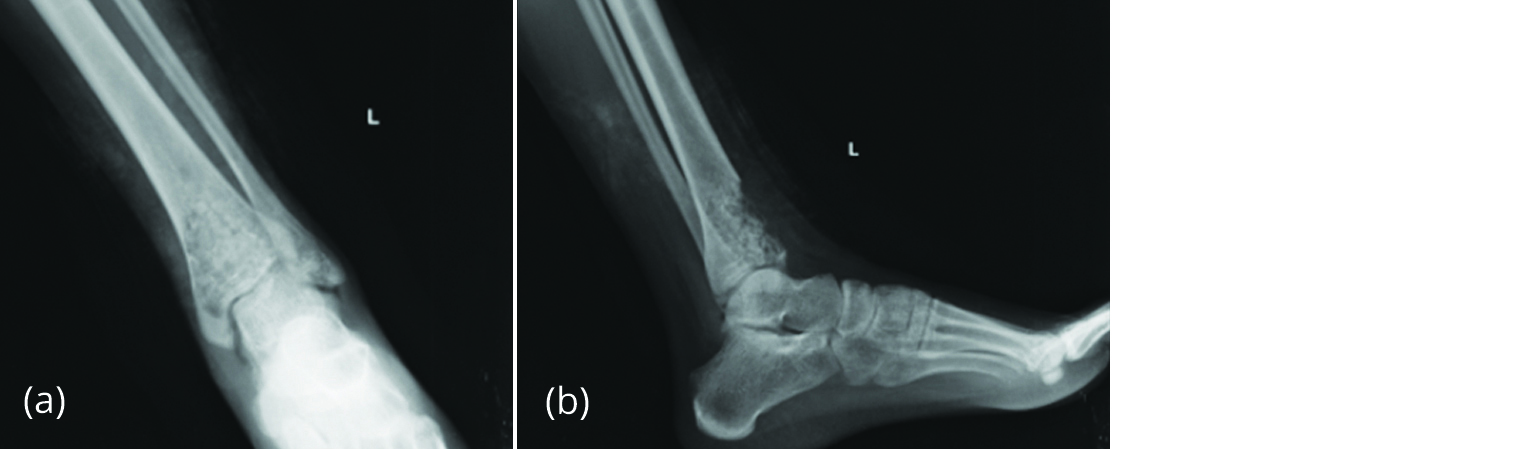
Figure 11a, b: Post-operative radiograph with cancellous bone graft in the cavity.
Patient developed pain 5 months after surgery. Radiograph of left ankle (Figure 12) showed absorption of graft and increase in size of lytic lesion suggesting recurrence of giant cell tumour.
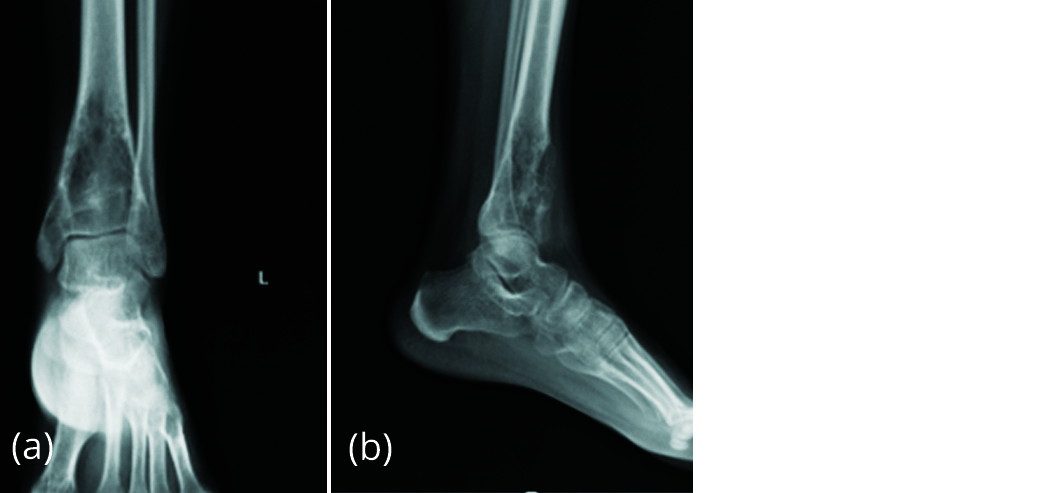
Figure 12a, b: Radiograph of left ankle with increase in size of lytic lesion.
With the experience we had with previous case and as recurettage does not guarantee no recurrence, patient was treated with injection denosumab 120mg with loading doses on day 1, 8, 15, 30 and later for every 4 weeks for 6 months.
Patient’s rest pain reduced after 2 weeks. After 6 months of denosumab therapy, patient had no pain, radiograph (Figure 13) revealed sclerosis, and restoration of anterior cortical continuity. Patient was able to bear weight fully.
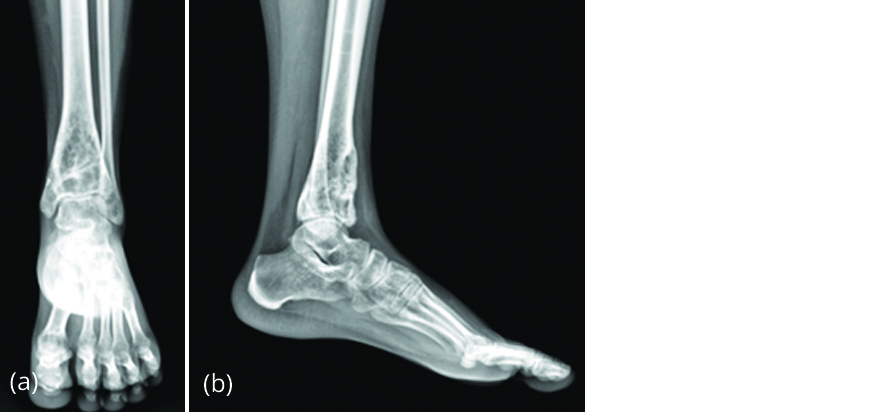
Figure 13a, b: Radiograph after 6 months of denosumab therapy with decrease in size of lesion and new bone formation.
Patient is under further follow up.
Discussion
Giant cell tumour of bone (GCTB) usually involves ends of long bones with fused physis, most common site being around knee (Distal femur and proximal tibia). Though benign, it is an aggressive osteolytic tumour with a challenging chance of local recurrence after surgical treatment. However, surgery is still the mainstay of treatment to start with. If the tumour is very aggressive, there may be local recurrence in spite of an effective extended curettage.
In spite of the fact that GCTB is much more common in south India at 30% of primary bone tumours [1] as compared to only 5% in the USA, there is not much published literature from this part of the country regarding an effective treatment of recurrence of the tumour.
Recent understanding of the pathogenesis of GCTB was responsible for the development of new treatment for this locally destructive tumour. The three main cellular components of GCTB are multi-nucleated osteoclast like giant cells, mono-nuclear spindle like neoplastic stromal cells and mono-nuclear cells of the monocyte/macrophage lineage. The neoplastic stromal cells secrete a cytokine “Receptor Activator of Nuclear factor Kappa B Ligand” (RANKL). The giant cells express a key mediator in osteoclastogenesis - the RANK receptor. The RANKL/RANK interaction is predominantly responsible for the extensive bone resorption by the tumour [8]. The discovery of RANKL/RANK pathway has led to the development of denosumab, a fully human monoclonal antibody [9]. Denosumab specifically binds to RANKL, thus preventing the RANKL/RANK interaction. The differentiation and functioning of giant cells is prevented, minimizing osteolysis and allowing normal osteogenesis to overtake.
We report our experience with denosumab in two cases of recurrent GCTB. In the first case of second recurrence of GCTB after extended curettage, usage of adjuvants (H2O2 and liquid nitrogen) and bone grafting, post denosumab therapy for 6 months there was considerable new bone formation on radiology. We were inquisitive as to the contents of the cavity left behind after denosumab therapy and we curetted the cavity. Histology of the contents showed immature trabecular bone and fibrous tissue. Neither giant cells nor stromal cells were seen. In a case report Maharaj et al. [10] have shown significant reduction of giant cells and stromal cells after denosumab therapy for 6 months. Proliferative densely cellular tumour stromal cells were replaced with non-proliferative differentiated woven new bone. This is consistent with our observation. According to Branstetter et al. [11], denosumab therapy results in conversion of cellular proliferative tissue into non-proliferating fibrous tissue and woven bone formation. However, Muller et al. [12] have shown viable tumour cells in the denosumab induced bone formation. According to Gaston et al. [13], denosumab has minimal inhibitory effect on stromal cells. This is in contrast to our observation where even the stromal cells were absent after denosumab therapy. However, the mechanism of action by which the stromal cells have been reduced is not understood clearly.
In the second case there was dramatic relief of pain and the patient was able to bear weight after new bone formation. However, we did not attempt at histological evidence of lack of tumour cells. Denosumab use in giant cell tumours not treated by surgery is yet to be defined [13]. Denosumab was approved by the US FDA for treatment of unresectable giant cell tumours or if surgery leads to significant morbidity [14]. Since the recurrence rate of GCTB after extended curettage is only 8% [7], we feel that denosumab need not be used in all cases immediately after extended curettage and is not cost effective. Denosumab usage may be restricted to only recurrences after extended curettage.
As on today there are many unanswered questions regarding denosumab therapy. The optimal dose is not defined. There are no definite guidelines on the duration of denosumab therapy [13, 15]. Stadler et al. [16] used denosumab in a case of recurrent GCTB for 2 years without any symptoms of toxicity. In both of our cases we stopped administration of denosumab after 6 months. We need to follow them for 2-3 years to be certain about recurrence. The incidence of uncommon complications like hypophosphatemia, osteonecrosis of Jaw and atypical Femoral fractures following long term denosumab therapy is unclear [14, 15]. We need to study more number of cases with a longer follow up to be definitive about the usage of denosumab.
Conclusion
Denosumab therapy is a fair option in treating giant cell tumour of bone recurring after an extended curettage. It need not be used routinely in all cases of GCTB after an extended curettage as recurrence rate is only 8%. Optimum dosage, duration of therapy and safety profile of denosumab needs to be ascertained by studying good number of cases with a longer follow up.
Acknowledgements
The Department of Radiology & Imageology, Krishna Institute of Medical Sciences (KIMS), Secunderabad-500003, Telangana, India.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Shanmugam S, Govindasamy G, Susikar S, Mani JG. Clinical outcome of Giant cell tumor of bone in South Indian population. Indian J Orthop Surg. 2017; 3(2):202–208.
[2] Balke M, Ahrens H, Streitbuerger A, Koehler G, Winkelmann W, et al. Treatment options for recurrent giant cell tumors of bone. J Cancer Res Clin Oncol. 2009; 135(1):149–58.
[3] Becker WT, Dohle J, Bernd L, Braun A, Cserhati M, et al. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. J Bone Jt Surg - Ser A. 2008; 90(5):1060–1067.
[4] Malek F, Krueger P, Hatmi ZN, Malayeri AA, Faezipour H, et al. Local control of long bone giant cell tumour using curettage, burring and bone grafting without adjuvant therapy. Int Orthop. 2006; 30(6):495–498.
[5] Dürr HR, Maier M, Jansson V, Baur A, Refior HJ. Phenol as an adjuvant for local control in the treatment of giant cell tumour of the bone. Eur J Surg Oncol. 1999; 25(6):610–618.
[6] Xu SF, Adams B, Yu XC, Xu M. Denosumab and giant cell tumour of bone-a review and future management considerations. Curr Oncol. 2013; 20(5):442–447.
[7] Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Jt Surg. 1987; 69(1):106–114.
[8] López-Pousa A, Broto JM, Garrido T, Vázquez J. Giant cell tumour of bone: new treatments in development. Clin Transl Oncol. 2015; 17(6):419–430.
[9] Singh AS, Chawla NS, Chawla SP. Giant-cell tumor of bone: Treatment options and role of denosumab. Biol Targets Ther. 2015; 9:69–74.
[10] Maharaj R, Panigrahi S, Das B. Effect of denosumab in giant cell tumor of bone. Oncol J India. 2017; 1(2):34–36.
[11] Branstetter DG, Nelson SD, Manivel JC, Blay JY, Chawla S, et al. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Cancer Res. 2012; 18(16):4415–4424.
[12] Müller DA, Beltrami G, Scoccianti G, Campanacci DA, Franchi A, et al. Risks and benefits of combining denosumab and surgery in giant cell tumor of bone-a case series. World J Surg Oncol. 2016; 14(1):1–7.
[13] Gaston CL, Grimer RJ, Parry M, Stacchiotti S, Dei Tos AP, et al. Current status and unanswered questions on the use of denosumab in giant cell tumor of bone. Clin Sarcoma Res. 2016; 6(1):13–18.
[14] Chawla S, Blay JY, Rutkowski P, Le Cesne A, Reichardt P, et al. Denosumab in patients with giant-cell tumour of bone: a multicentre, open-label, phase 2 study. Lancet Oncol. 2019; 20(12):1719–1729.
[15] Lipplaa A, Dijkstra S, Gelderblom H. Challenges of denosumab in giant cell tumor of bone, and other giant cell-rich tumors of bone. Curr Opin Oncol. 2019; 31(4):329–335.
[16] Stadler N, Fingernagel T, Hofstaetter SG, Trieb K. A recurrent giant cell tumor of bone treated with denosumab: A case report. Clin Pract. 2015; 5(1):4–5.