Full Text
Hospital Infection Control Committee (HICC) play an important role in conducting the various activities which are important for the patient, attendant and health care workers. The Committee members discuss and prepare policies regarding the practice of infection control and strategies for surveillance, prevention & control of health care associated infections, antimicrobial resistance and related events in healthcare settings.
The National Accreditation Board of Health (NABH) standards provide framework for quality assurance and quality improvement for hospitals. The standards focus on patient safety and quality of care. The standards call for continuous monitoring of sentinel events and comprehensive corrective action plan leading to building of quality culture at all levels and across all the functions. The NABH manual Chapter 5 deals in detail with Hospital Infection Control (HIC). The chapter has 9 standards and 52 objective elements [1]. It gives lot of emphasis on fulfilling criteria as per CDC guidelines, WHO guidelines, Ministry of Health and Family Welfare, Govt. of India.
Hospital Infection Control Committee (HICC) have a team of multidisciplinary members.
Hospital Infection Control activities are a team work with coordinated activities from various departments in the hospital. The various activities are conducted by making use of a planner for the year. It has defined activities periodically. The Microbiologists & Infection Control Nurse (ICN), under the guidance of Chairman, interact with various departments in the hospital and conduct these activities. They are
Hospital Infection Control Committee (HICC) Meeting
The HICC has members of multidisciplinary specialties. The team consists of a Chairman, senior hospital administrator, an orthopedic surgeon, a senior microbiologist as a member secretary, a surgical gastroenterologist, a physician, a pulmonologist, a microbiologist, three intensivists, a senior infection control nurse (ICN) and a junior infection control nurse, nursing superintendent, sister i/c of operation theatre. Representation from the department of Quality, Maintenance, Biomedical, F&B, House Keeping I/C is also present. The committee members prepare policies & procedures for various departments in the hospital regarding preventing and reducing the risk of nosocomial infections & monitoring outbreak of any infection [2]. The committee members have a meeting once in a month. The schedule of the meeting is 3rd Wednesday of every month. In the meeting the agenda is discussed and the minutes of the meeting is submitted to the department of Quality.
Infection control team (ICT) meeting
The infection control team is responsible for day to day functioning of infection control programme. They conduct surveillance & detect outbreaks [3]. They participate in infection prevention and control activities on a daily basis. A total of 28 ICT’s are present. It has a network with at least one person from each ward or department in the team. 17 ICT are present in ICU’s: MICU I & II, ICCU, CTICU, SDCTICU, SICU, NSICU, PICU, NICU, PCTICU, SDPCTICU, CMC, OT, Dialysis, Labor Room, KTU, Casualty and Blood Bank. 11 ICT’s are present in the ward. In each team a doctor and sister/brother is present. The responsibility to conduct the meeting is on the sister/brother. The schedule for the meeting is : 2nd Friday. A form is issued by the ICN to all the sister/brother I/C to inform the infection in their unit. After entering the details, the checklist is submitted to the ICN, which will be analyzed. The report is submitted in the HICC meeting.
Post fumigation swabs of operation theatres
All the operation theatres are disinfected daily by using Bacillocid after each surgical procedure and also end of the day. Fumigation is done weekly. Every fortnight post fumigation swabs are collected and tested for bacterial growth of aerobic and anaerobic organisms. 7 swabs are collected from designated places (boyles, light, ac, volve, floor, table and suction) in an operation theatre and processed for bacterial growth. Settle plates are used to check the sterility of air in the operation theatre (both bacterial & fungal growth).
Random swab collection of any two operation theatres
The purpose of collecting post fumigation swabs form operation theatres, randomly, is to check sterility of the operation theatre at any given time. Every week, on Wednesday, swabs are collected from any 2 operation theatres & tested for bacterial growth.
Central sterile supply department (CSSD)
In the CSSD, Steam sterilizers and Ethylene oxide sterilizers are used. As a quality check, Bowie Dick test is performed on a daily basis. Use of biological indicators (bacterial spores) is done once a week i.e., on every Monday. The vial containing spores are used as per the standard recommendation.
For steam sterilizers the bacterial spores of ATCC 7953 Bacillus sterothermophilus are used which requires an incubation of 57°C± 2°C. It is incubated (Figure 1) in a Browne incubator. The color of the vial is purple in color. It is observed at 8,12,24 & 48 hours. If the spores have been killed in the sterilization cycle the purple color does not change, indicating adequate sterilization. If the spores survive the sterilization cycle, the purple color does change from purple to yellow indicating inadequate sterilization (failure in sterilization) [4].

Figure 1: Incubator.
For ETO sterilizers the bacterial spores of ATCC 9372 Bacillus atrophaeus are used which requires an incubation of 37°C± 2°C. It is incubated in an incubator at 37°C. The color of the vial is red in color. It is observed at 8,12,24 & 48 hours. If the spores have been killed in the sterilization cycle the red color does not change. If the spores survive the sterilization cycle, the red color does change to yellow indicating inadequate sterilization (failure in sterilization).
ICU post disinfection or fumigation swabs
All the ICU’s are fumigated or disinfected regularly to avoid hospital acquired infections in the patients [5]. The instruments and surfaces are cleaned and disinfected with disinfectants regularly. Fumigation is done as per the standard recommended procedure. When the occupancy of the ICUS is full then disinfection is done. Post fumigation or disinfection swabs are collected and checked for bacterial & fungal growth.
Bacteriology of drinking water
Drinking water has to be clear, colorless and without disagreeable taste or odor. It should be free from contamination. All the drinking water supplies are checked for contamination. Every month, in the 3rd week testing is done. Rapid PA coliform test kit is used. It detects the presence or absence of coliform bacteria in drinking water. It is a simple modification of Multiple Tube Procedure [6]. It is presumptive test for coliforms. The principle of the test is : the medium contains nutrient required for growth of coliforms. Lactose is fermented. Sodium lauryl sulphate inhibits organisms other than coliforms. A quality control check is done by using the ATCC 25922 Escherichia coli. If the medium changes from purple-red to yellow, it indicates presence of coliforms. Reporting is done after 48 hours. No color change indicates absence of coliforms & the water is safe for drinking purpose.
Methicillin resistant Staphylococcus aureus (MRSA) screening of staff for carrier state
Screening of Health Care Workers for MRSA carrier state is done to prevent infection in patients [7]. The infection can cause morbidity, prolonged stay in the hospital leading to economic burden to the patient. It may lead to mortality. Hence to prevent infection to be transmitted to patients, the carrier state in HCW’s is screened. The HCW in high risk areas like ICU’s, OT, Cath lab, Dialysis unit, Labor room & KTU are screened. The schedule is every year. 2 swabs are collected from each HCW, one from anterior nares and one the other from the fingers. It is inoculate on a special medium Mannitol Salt Agar. Staphylococcus aureus produce yellow color colonies (Figure 2). A quality control stain ATCC 29213 Staphylococcus aureus (MSSA) & ATCC 43300 Staphylococcus aureus (MRSA) is inoculated to check the performance.
Use of Mupirocin ointment for 5 days is advised for the carriers. A post treatment repeat swab is collected & checked for MRSA growth.
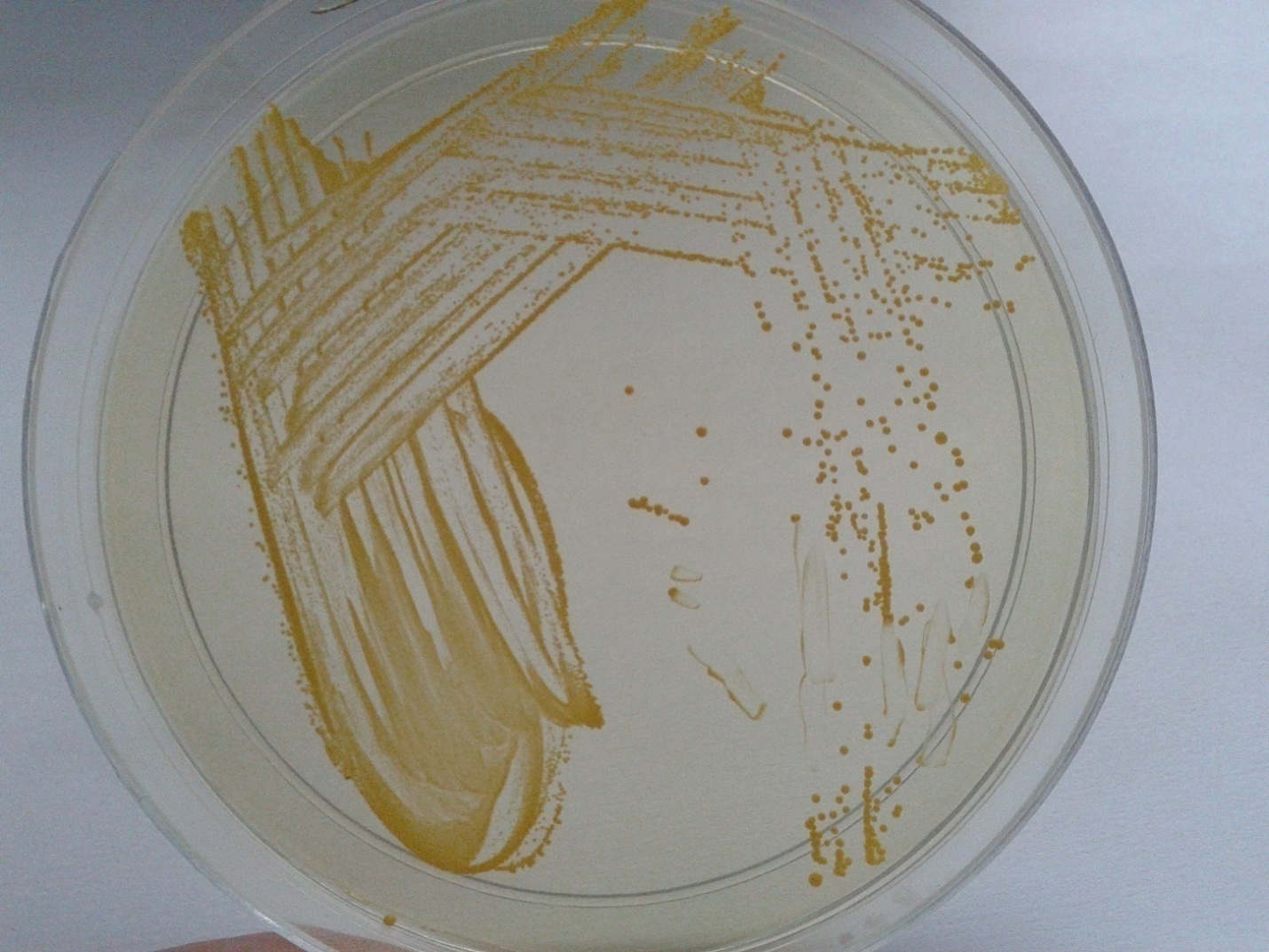
Figure 2: Mannitol salt agar showing yellow colored colonies of Staphylococcus aeureus.
Screening of canteen (F& B) staff- stool ova & cyst: Once in a year
Canteen (F& B) staff are screened for ova & cyst in the stool. If any HCW is found carrier then a complete treatment is given Repeat samples are checked.
Vaccination of staff- HBsAg
HCW’s are vaccinated for Hepatitis B virus (HBV). The schedule A is followed. Schedule A is recommended for those who come under high-risk category. 3 doses of Hepatitis B vaccine (HBV) is given. Vaccination is done on 2nd of every month.
As per Schedule A: 1st dose: At an elected date, 2nd dose: 1 month after the 1st dose, 3rd dose: 2 months after the 1st dose (0,1 & 2 months). A booster dose is recommended 12 months after the 1st dose. The protective level of anti-HBs is 10mIU/ml. A second booster dose may be required after 8 years if the titers fall below 10mIU/ml.
Effluent check of laundry for pathogens
Dirty linen in hospital contain microorganism like Staphylococcus aureus, Pseudomonas aeruginosa. During the process of washing these organism are destroyed by the action of soap & bleach. The effluent should not contain any of these organism indicating the various steps in the washing procedure are correct. In the laundry two washers are used. Every month, both the instruments effluent water is checked for pathogens like Staphylococcus and Pseudomonas.
Infection control bulletin
An Infection Control Bulletin, containing information on various aspects on infections is released. It provides information to all the Health Care Workers. One issue is released every two months. Till now 34 issues have been released.
Antibiotic sensitivity data & antimicrobial stewardship committee
An in house data is generated. The patient samples, which show growth of organism, are noted on a daily basis. The sensitivity pattern of the bacterial & fungal culture growth is noted by the ICN in the Microbiology laboratory. Then it is compiled for one month. It is analyzed by the Microbiologist. 4 months data is compiled as 40-45 pages book, submitted and discussed in the HICC meeting. It is distributed to all the I/C Sisters and Brothers in the ICU’s and wards. It has all the necessary information like the predominant organisms & the antibiotic sensitivity pattern in the respective ICU’s /wards that cause infection. It helps in starting empirical therapy to the patient & reducing misuse of antibiotics.
Every year, Antimicrobial Sensitivity Protocol is prepared from 1 year culture positive data (Figure 3). It is prepared by the ICN & analyzed by the Microbiologists. Then it is discussed by the Antimicrobial Stewardship Committee members. After the data is approved by the committee, it is released as a pocket guide. It is made available in all ICU’s & wards. The pocket guide has a validity period of one year. Till now 4 pocket guides have been released.

Figure 3: Antibiotic protocol pocket guide-KIMS.
The members of Antimicrobial Stewardship Committee include two microbiologists, a physician, one pulmonologist, pediatrician & ICN. The Antimicrobial Sensitivity Protocol is released by Antimicrobial Stewardship Committee [8].
The primary goal of antimicrobial stewardship is to optimize clinical outcomes while minimizing unintended consequences of antimicrobial use and emergence of resistance.
Anti HBs levels
The occupational safety & Health Administration (OSHA) requires that Hepatitis B vaccine be given to HCW who are exposed to risk of blood & body fluids. HCW’s in high risk areas like all ICU’s , OT staff, Dialysis staff, Cath Lab & Blood bank are screened for anti-HBs levels who were completely vaccinated for hepatitis B virus (HBV). Every 3 years estimation of anti-HBs levels is done to know the success of Hepatitis B vaccination for the HCW’s . The samples of the HCW’s are processed in the Instrument Architect. The technology involved is Chemiluminescent Microparticle Immunoassay (CMIA) .The anti-HBS concentration >10.0 mIU/ ml indicates protective levels of antibody. The sensitivity is 97.54 % & specificity is 99.67%.
HIC activities are a team work of various department of the hospital. It involves funding from the management for smooth functioning of all the above mentioned activities.
Conflicts of interest
Authors declare no conflicts of interest.